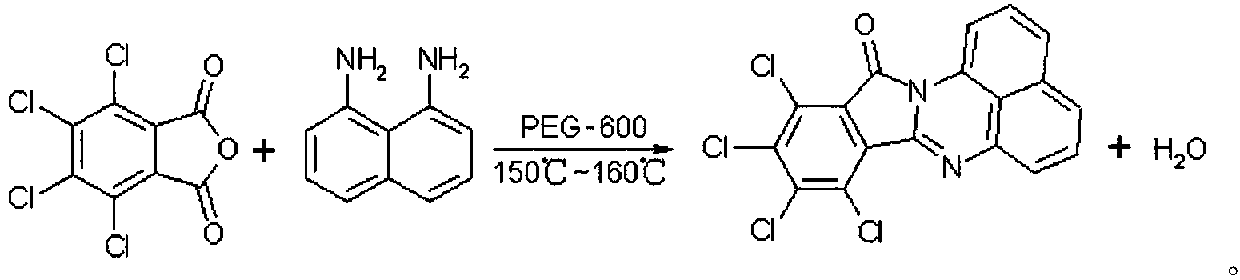
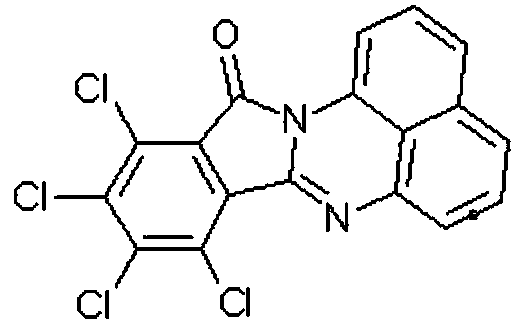
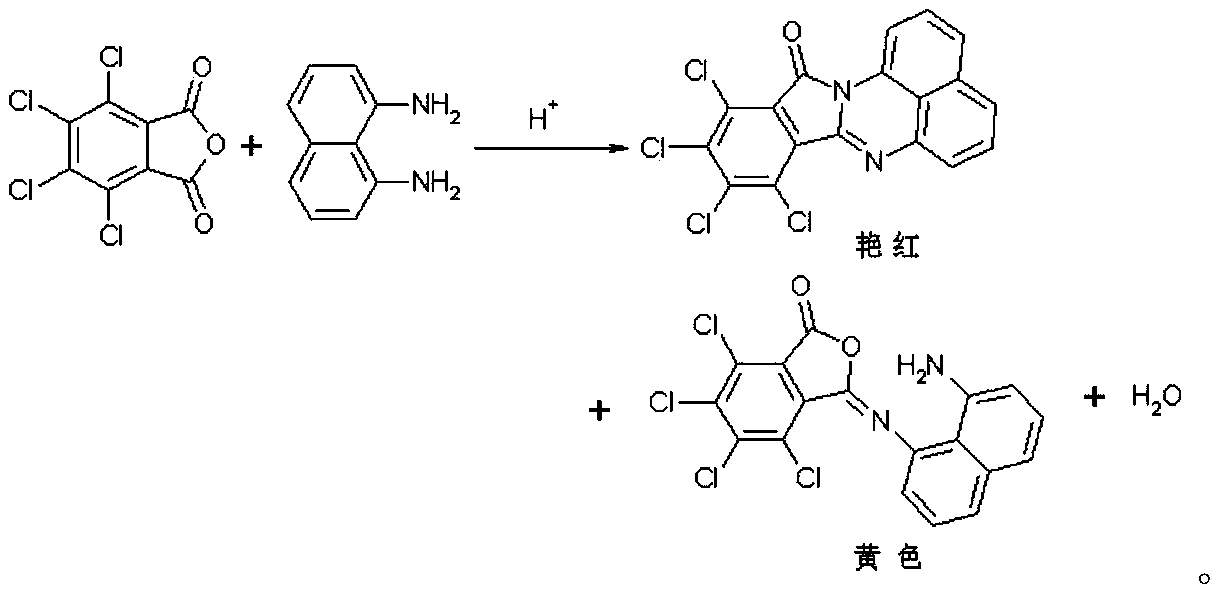
Synthetic method of red naphthalene cyclic ketone dye
A synthesis method and a technology of naphthocyclic ketone are applied in the manufacturing field of chemical dyes, which can solve the problems of low solvent recovery rate, high price and high solvent price, and achieve the effects of good shade and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The o-dichlorobenzene of 150g and the tetrachlorophthalic anhydride of 50g are dropped into the reactor that has water separator and reflux condenser, after stirring for 0.5h, add 27.8g of 1,8-diaminonaphthalene and 4.5g of PEG-600 and Start to heat up and turn on the circulating water at the same time. When the temperature rises above 150°C, heat preservation starts, followed by HPLC. After 5.5 hours, the heat preservation ends, and the temperature is lowered to 80°C. It is isolated with 37.5g of methanol, and after the separation is completed, it is lowered to 50°C. Suction filtration, the filter cake is soaked in a small amount of methanol, drained, and again Wash with a small amount of methanol, drain, discharge, and vacuum-dry the filter cake at 50°C for 24 hours to obtain 66.3g of the product, with a yield of 93%. Compared with the standard product, the color and intensity are as follows:
[0023] △E*
[0024] Among them, △E* is the total color difference...
Embodiment 2
[0026] Put 200g of o-dichlorobenzene and 50g of tetrachlorophthalic anhydride into the reactor with water separator and reflux condenser, add 27.8g of 1,8-diaminonaphthalene and 6g of PEG-600 after stirring for 0.5h and start Raise the temperature and turn on the circulating water at the same time. When the temperature rises above 155°C, heat preservation starts, followed by HPLC. After 5 hours, the heat preservation ends, and the temperature is lowered to 80°C. Isolate with 50g of methanol. After the separation is completed, the temperature is lowered to 50°C. Suction filtration, the filter cake is soaked in a small amount of methanol, drained, and again with a small amount of Washed with methanol, drained, and discharged, the filter cake was vacuum-dried at 50°C for 24 hours to obtain 67.4g of the product, with a yield of 94.5%. Compared with the standard product, the shade and intensity were as follows:
[0027] △E*
Embodiment 3
[0029] 250g of o-dichlorobenzene and 50g of tetrachlorophthalic anhydride are dropped into a reactor with a water separator and a reflux condenser, and after stirring for 0.5h, 27.8g of 1,8-diaminonaphthalene and 7.5g of PEG-600 are added and Start to heat up and turn on the circulating water at the same time. When the temperature rises above 155°C, heat preservation starts, followed by HPLC. After 5 hours, the heat preservation ends, and the temperature is lowered to 90°C. It is isolated with 62.5g of methanol. After the separation is completed, it is lowered to 50°C. Suction filtration, the filter cake is soaked in a small amount of methanol, drained, and used again A small amount of methanol was washed, drained, and the material was discharged. The filter cake was vacuum-dried at 50°C for 24 hours to obtain 68.1 g of the product, with a yield of 95.5%. Compared with the standard product, the color and intensity were as follows:
[0030] △E*
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com