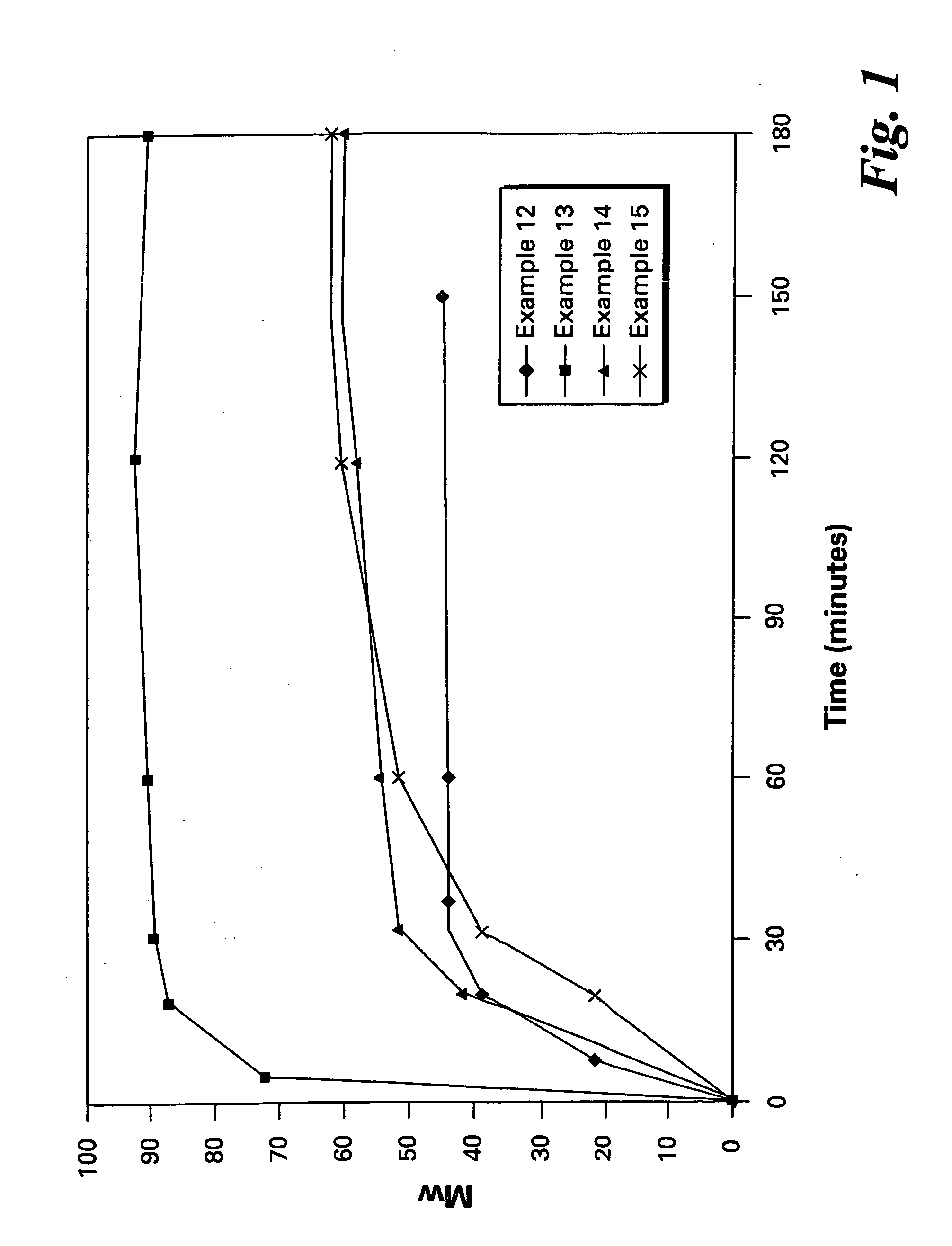
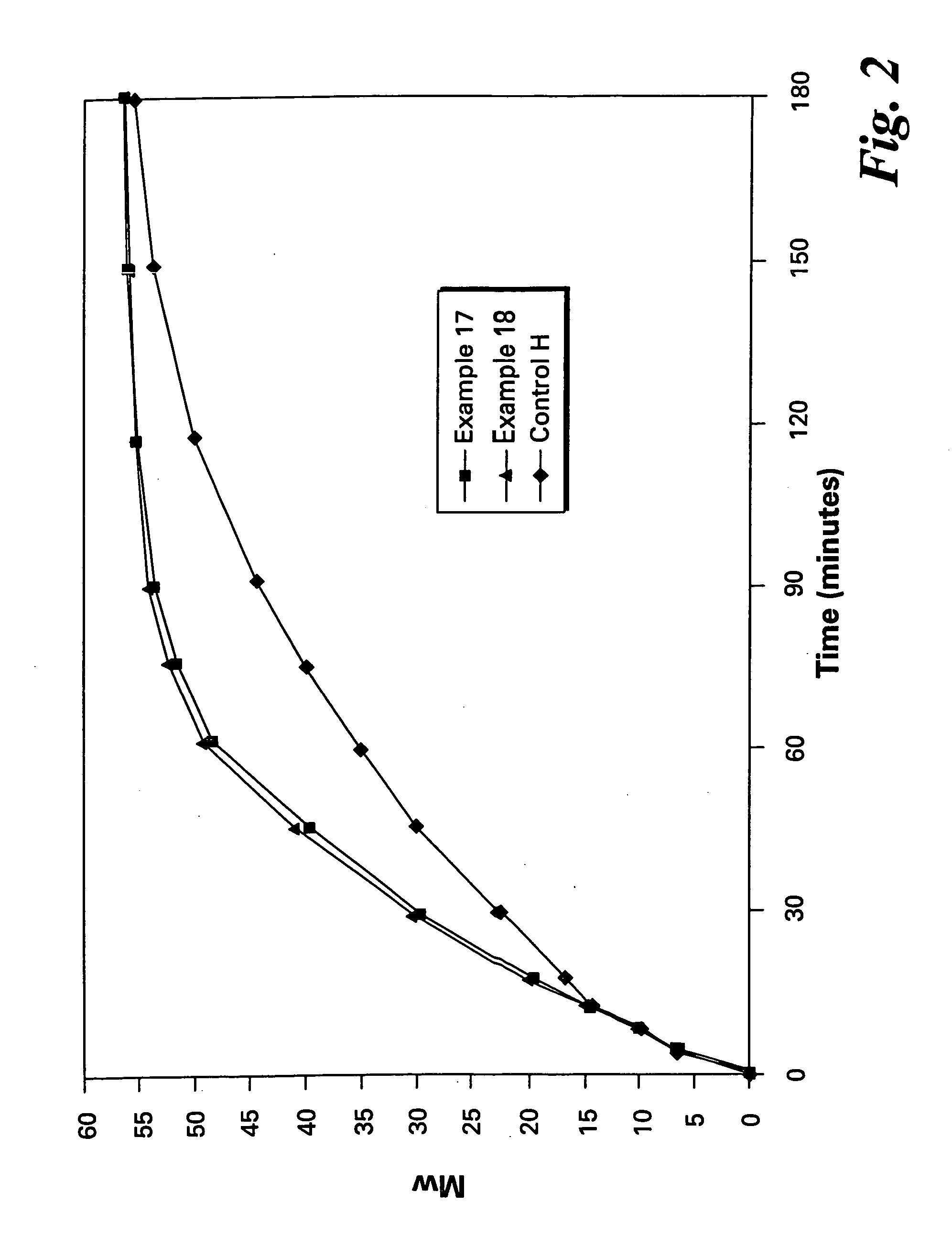
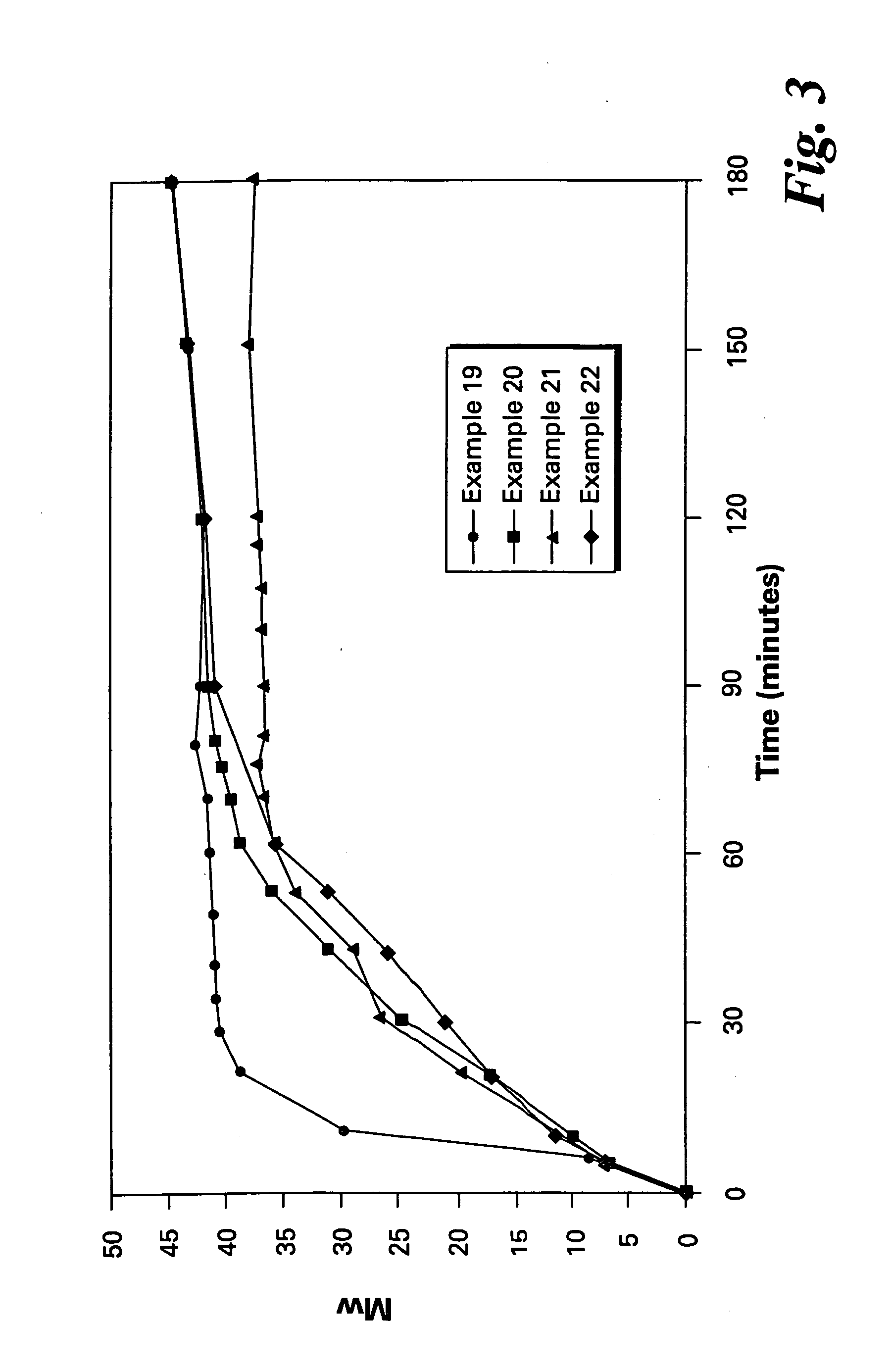
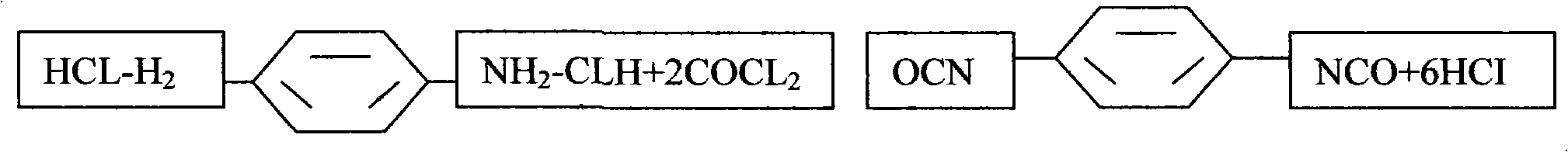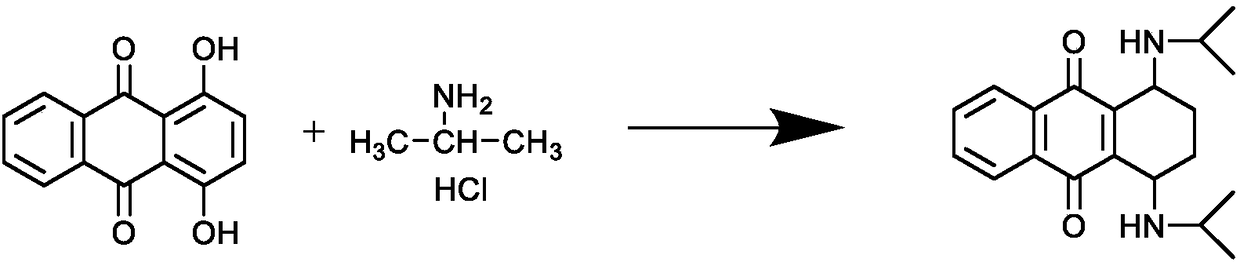Patents
Literature
47 results about "1,2-Dichlorobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
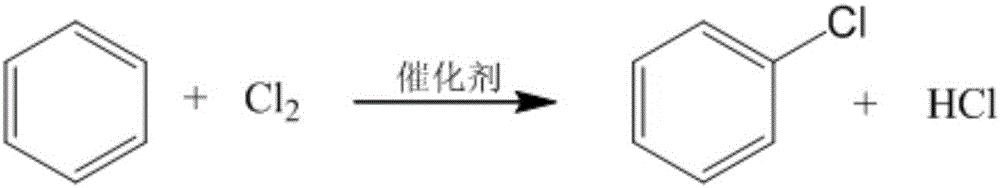
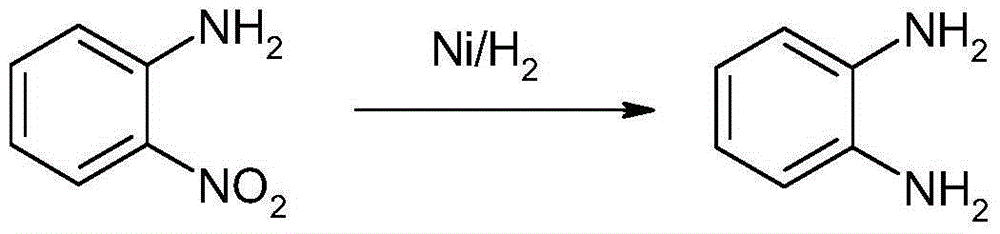
1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an organic compound with the formula C₆H₄Cl₂. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.
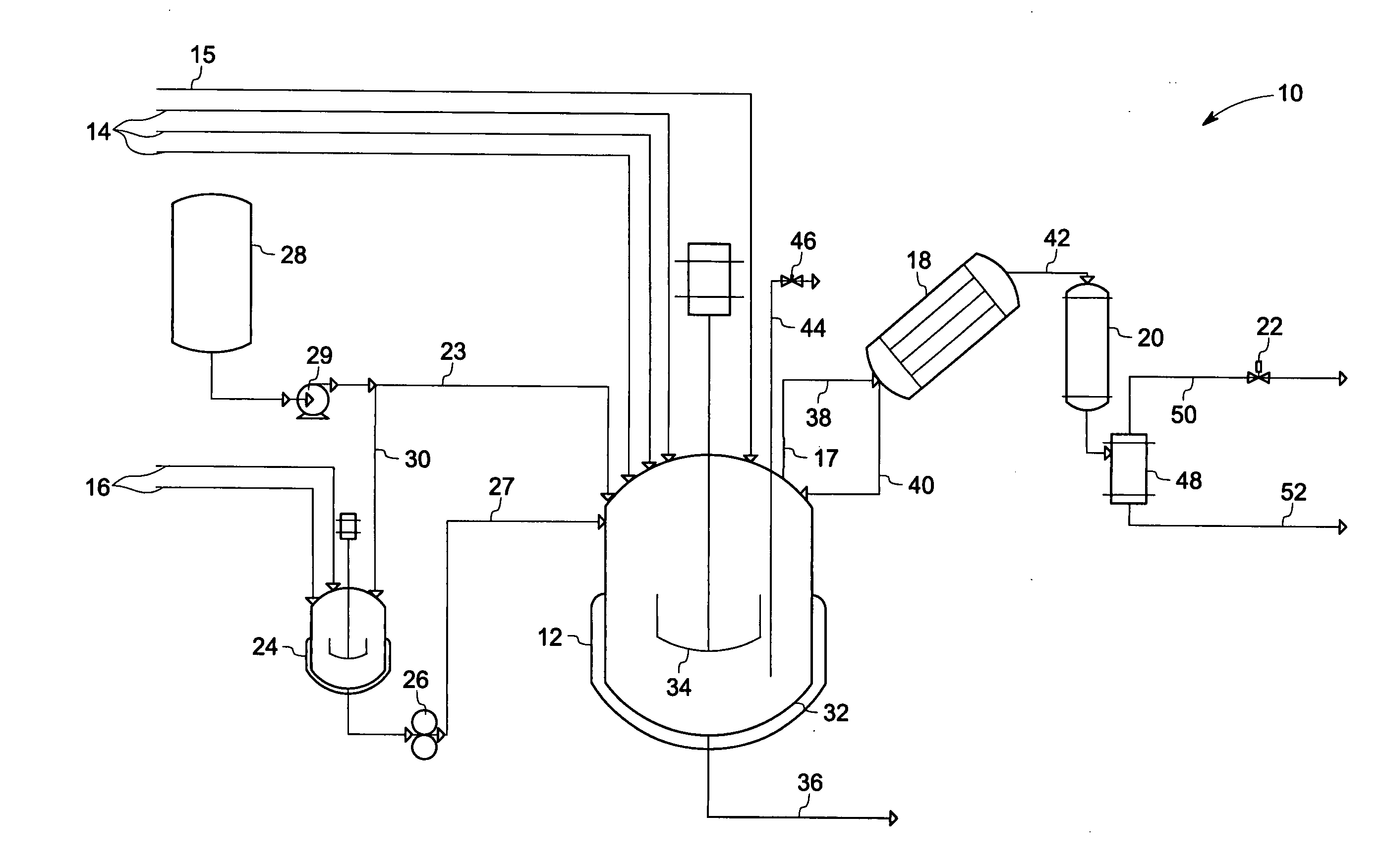
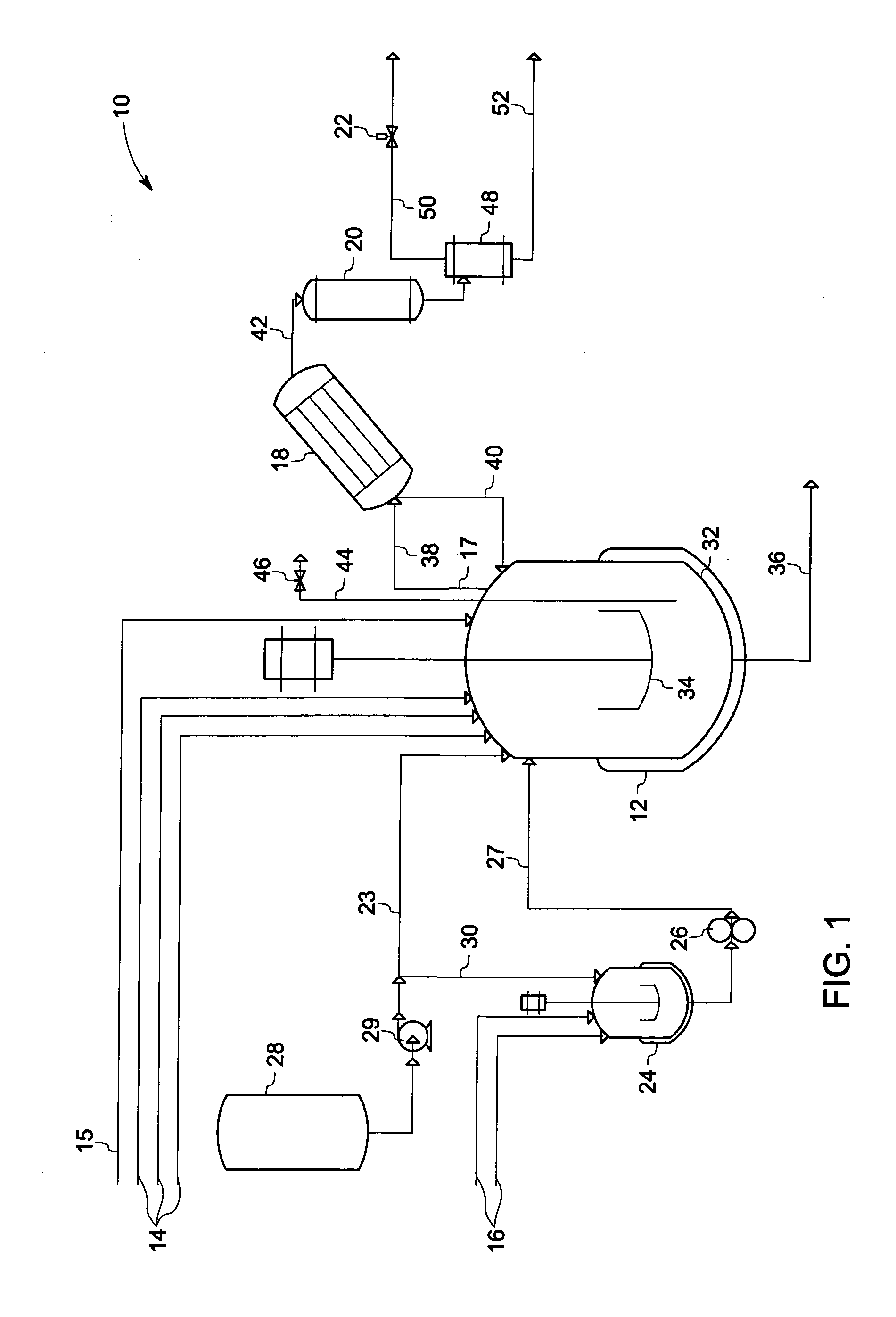
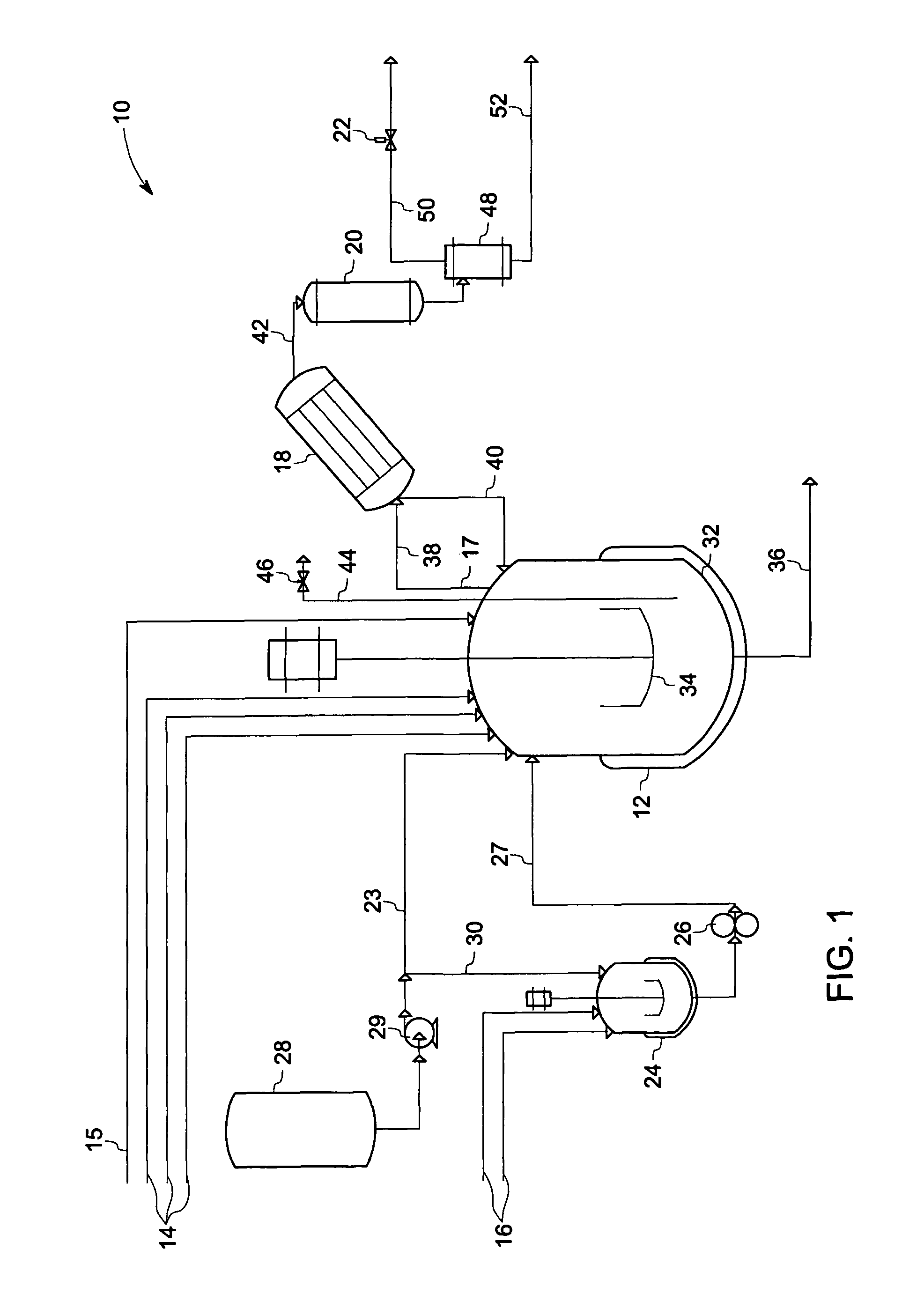
Phase transfer catalyzed method for preparation of polyetherimides
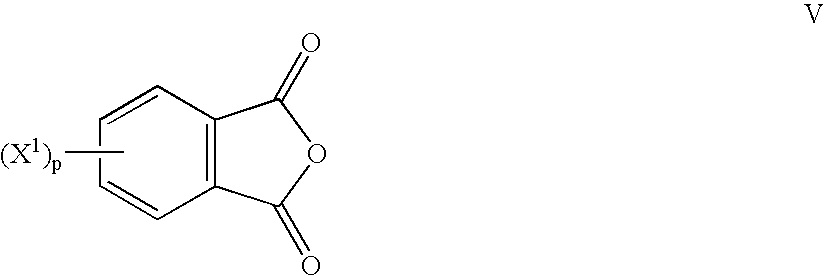
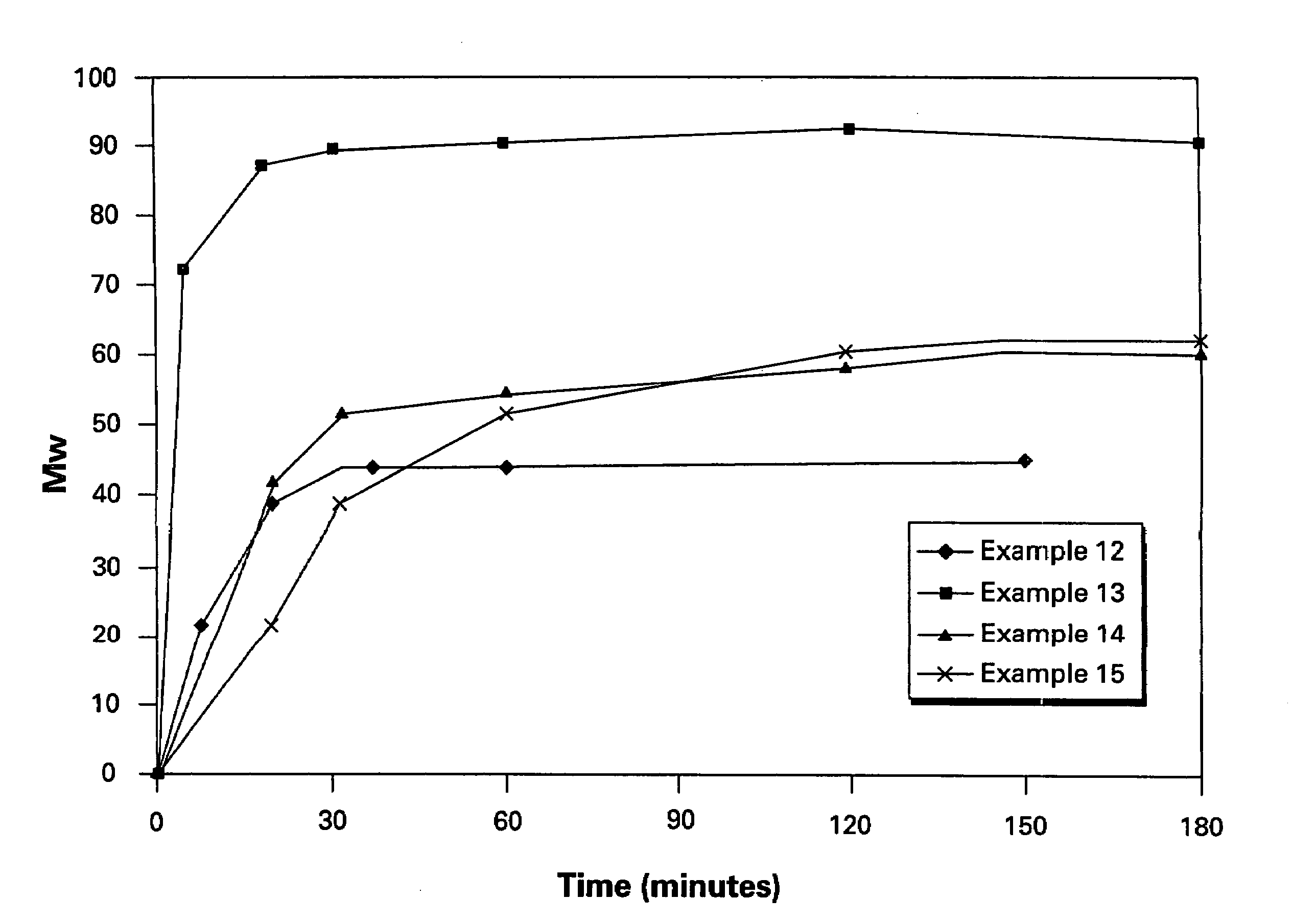
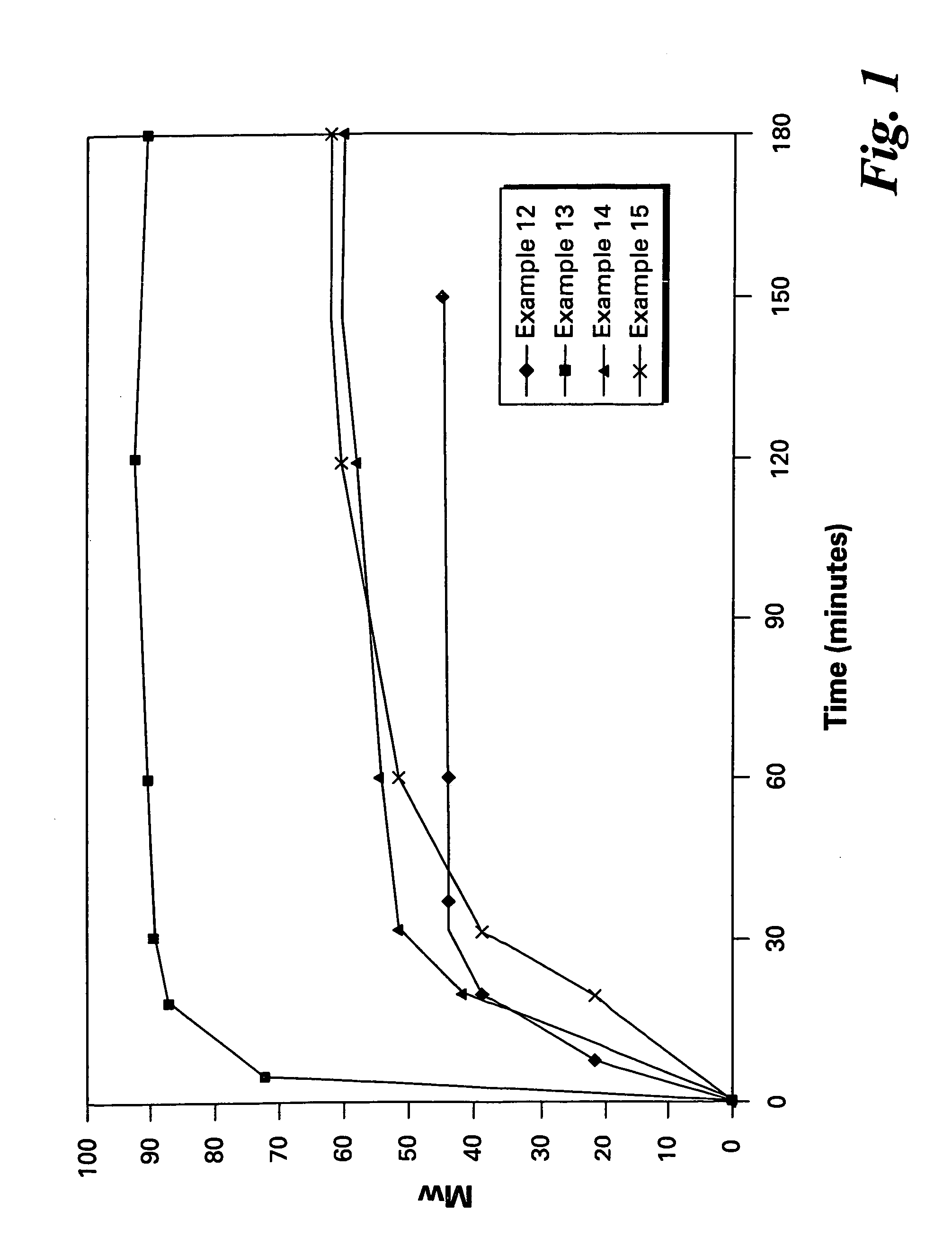
Polyether polymers, such as polyetherimides, are prepared by the reaction of a dihydroxy-substituted aromatic hydrocarbon alkali metal salt, such as bisphenol A disodium salt, with a bis(N-(chlorophthalimido))aromatic compound, such as 1,3- and / or 1,4-bis(N-(4-chlorophthalimido))benzene, in a solvent such as o-dichlorobenzene and in the presence of a phase transfer catalyst such as a hexaalkylguanidinium chloride. Several embodiments may be employed to improve the method. They comprise employing substantially dry reagents, employing a high solids level in solvent, beginning with an excess of bis(N-(chlorophthalimido))-aromatic compound and incrementally adding alkali metal salt, employing alkali metal salt of small particle size, and using reagents of high purity.
Owner:SHPP GLOBAL TECH BV
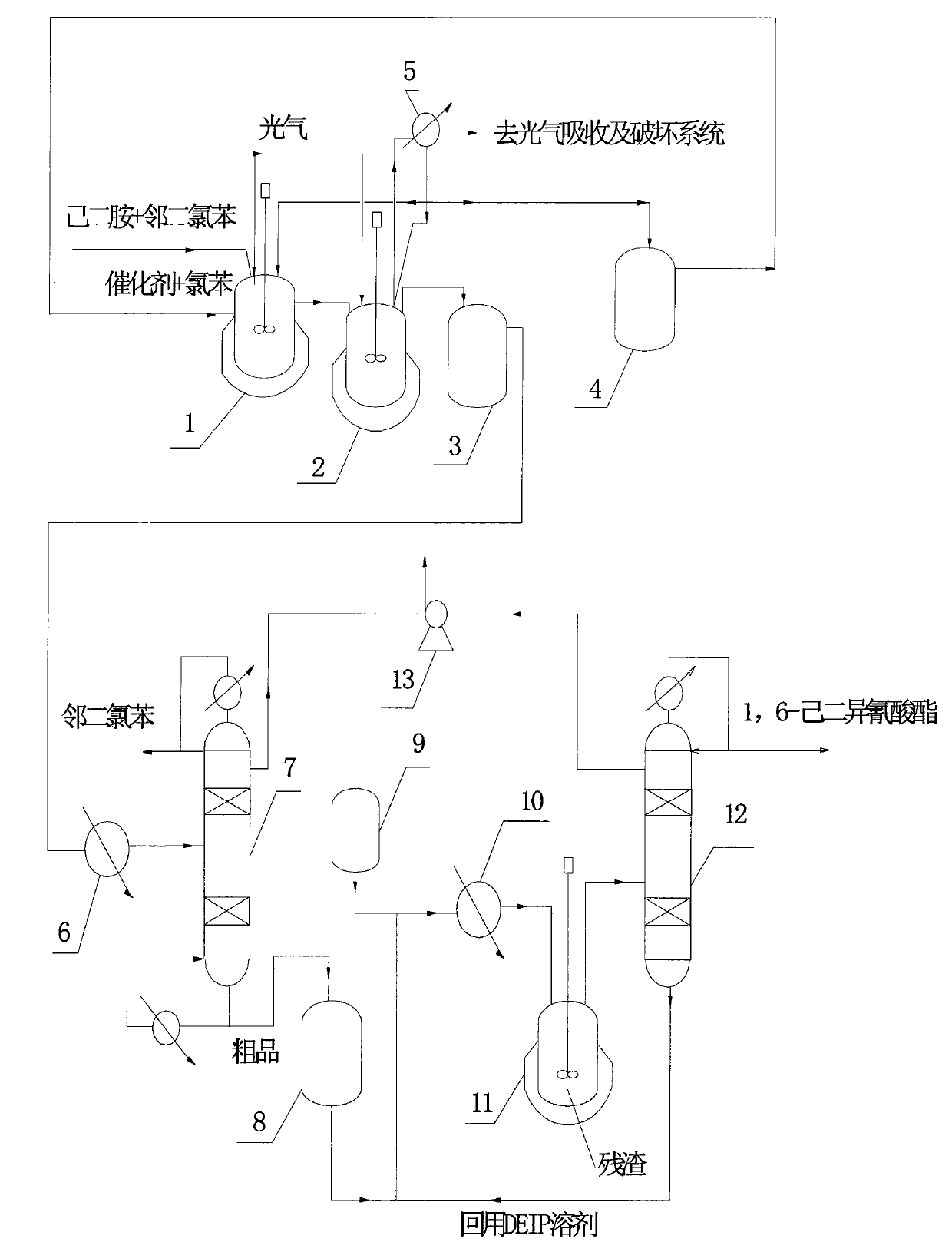
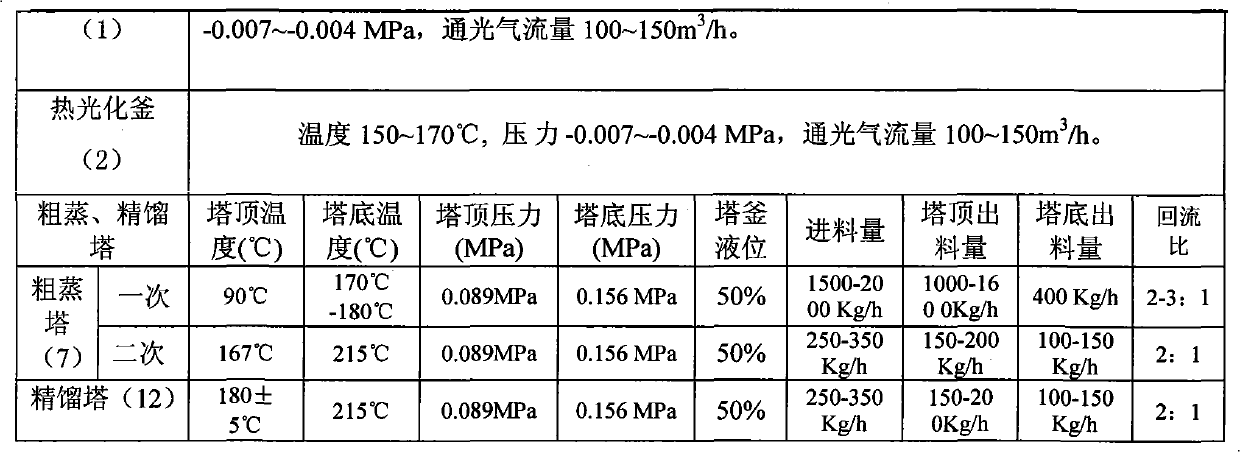
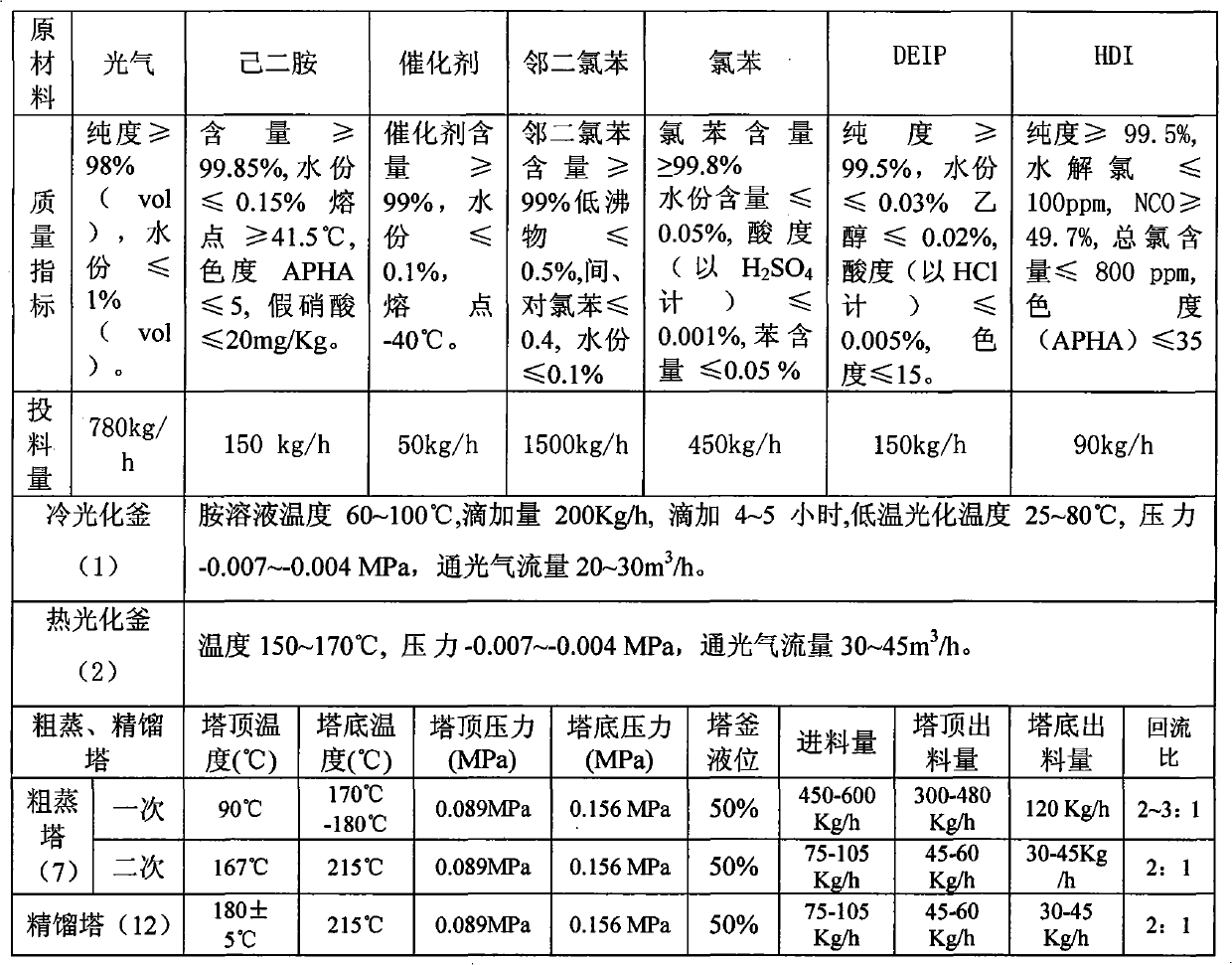
Method for continuously preparing 1,6-hexamethylene diisocyanate
InactiveCN101928235AOrganic compound preparationIsocyanic acid derivatives purification/separationChlorobenzeneBurn treatment
The invention relates to a method for preparing crude 1,6-hexamethylene diisocyanate through the phosgene method and separating pure 1,6-hexamethylene diisocyanate through rectification. The method uses o-dichlorobenzene and chlorobenzene as solvents and comprises the following steps: using hexanediamine and excessive phosgene to perform the two-step reaction of low temperature photochemical reaction and high temperature photochemical reaction under the action of catalyst and generate 1,6-hexamethylene diisocyanate (HDI), removing o-dichlorobenzene through distillation for reuse, continuously distilling to remove by-product chlorinated isocyanate, further mixing with DEIP solvent in a certain ratio through compulsory stirring, heating to perform flash evaporation and rectification, preparing the 1,6-hexamethylene diisocyanate product and DEIP through separation and purification, and regularly discharging the slagged tarry macromolecular compound to perform burning treatment.
Owner:甘肃银光聚银化工有限公司
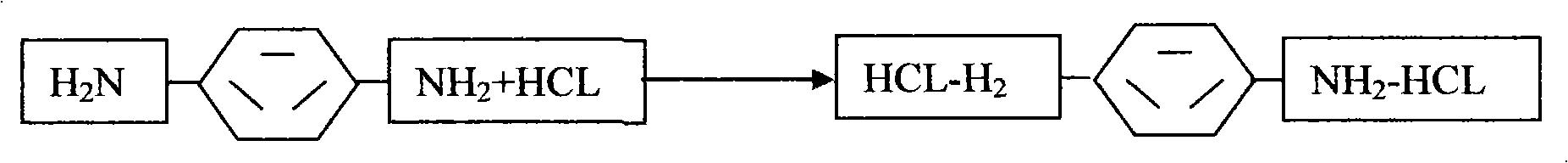
Novel process for preparing p-phenylene diisocyanate (PPDI) based on phosgene
InactiveCN101638372AEmission reductionIsocyanic acid derivatives preparationOrganic compound preparationPhosgeneRaw material
The invention provides a novel process for preparing p-phenylene diisocyanate (PPDI) based on phosgene, which relates to a process for preparing chemical raw materials, in particular to a novel process for preparing the p-phenylene diisocyanate (PPDI). The process comprises the following steps: 1, hydrochloric acid formation reaction; 2, salifying reaction; and 3, photochemical reaction which is to transfer a mixture solution of para-phenylene diamine dihydrochloride-o-dichlorohenzene to a photochemical kettle, control the temperature, introduce the phosgene in an infinite reflux state to react, determine that the reaction is terminated after reaction materials are settled, introduce quantitative nitrogen to remove acid, sample and analyze the reactant, and obtain the p-phenylene diisocyanate PPDI through rectifying, crystallizing, centrifugating, drying, packaging or refining, slicing and packaging when the acidity content is qualified. In the process, a reasonable salifying reactioncondition is customized to avoid slow reaction of a prepolymer, complicated reaction mechanism, and easy generation of side products and adverse factors influencing the reaction in the next step.
Owner:XINYI AGRI CHEM PLANT JIANGSU PROV
Method for preparing chlorobenzene, p-dichlorobenzene and o-dichlorobenzene in benzene chlorination
ActiveCN103819307AQuick responseIncrease production capacityHalogenated hydrocarbon preparationChlorobenzeneReaction temperature
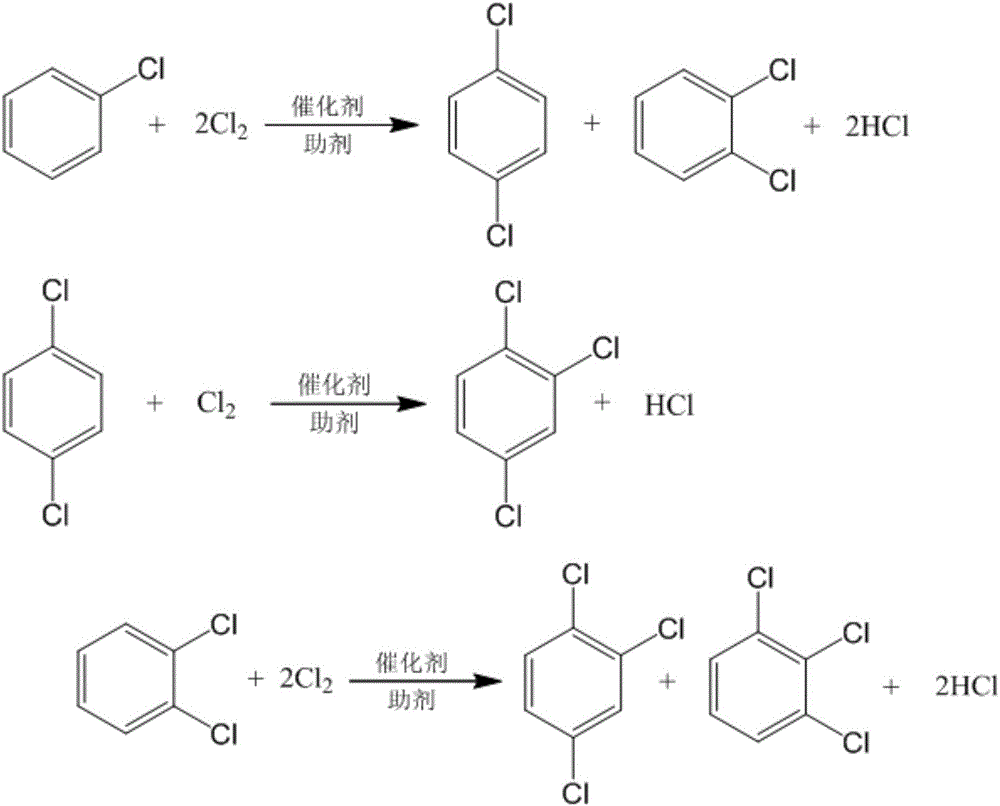
The invention discloses a method for preparing chlorobenzene, p-dichlorobenzene and o-dichlorobenzene in benzene chlorination, and relates to a preparation method of chemical raw materials. The method comprises the following steps: continuously introducing dry benzene and chlorine with a high activity and high p-dichlorobenzene selectivity catalyst into an outer circulation reactor, wherein the benzene and chlorine need sufficient retaining time, reacting at a set temperature to prepare p-dichlorobenzene, obtain o-dichlorobenzene at the same time and co-produce chlorobenzene, preparing dichlorobenzene by using the outer circulation reactor and taking the benzene and the chlorine as raw materials and metal sulfide as a catalyst, at the same time co-producing chlorobenzene, forming circulation of the materials through the introduced nitrogen and generated hydrogen chloride as power so as to obtain good stirring for prompting mixing, diffusion, heat conduction and mass transfer of the reactants. The method is rapid in reaction speed, high in capacity, large in ratio of p-dichlorobenzene and o-dichlorobenzene, free of polychlorobenzene byproduct, and high in flexibility and operation flexibility as the ratio of the chlorobenzene to the dichlorobenzene in a product can be adjusted according to demands.
Owner:LIAONING FANGDA ENG DESIGN CO LTD
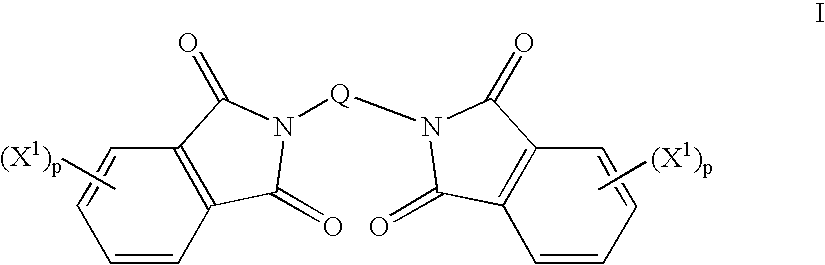
Method to prepare bis(haloimides)
Bis(halophthalimides) are prepared in mixture in an organic liquid such as ortho-dichlorobenzene or anisole, by a reaction at a temperatire of at least 150° C. between at least one diamine compound and at least one halophthalic anhydride in the presence of imidization catalyst. The reaction mixture is maintained at about 15% by weight solids content and rich in the halophthalic anhydride by constantly monitoring the reaction mixture using analytical methods such as high performance liquid chromatography. The product mixture may be directly employed in the direct preparation of polyetherimides, and similar slurries may be employed to prepare other polyether polymers.
Owner:SHPP GLOBAL TECH BV
Method for producing 2,4-dichloroacetophenone by using solid waste chlorobenzene tar as raw material
InactiveCN101898947ASolve your worriesAlleviate supply and demand pressureCarbonyl compound preparation by condensationAcetyl chlorideM-dichlorobenzene
The invention discloses a method for producing 2,4-dichloroacetophenone by using solid waste chlorobenzene tar as a raw material. The method mainly comprises the following steps of: extracting m-dichlorobenzene from the solid waste chlorobenzene tar, and then performing five steps of acylation, washing, distillation, crystallization and packing in the 2,4-dichloroacetophenone production process. The method is characterized in that: the acylation reaction comprises that: the m-dichlorobenzene and acetyl chloride undergo the acylation reaction under the action of anhydrous aluminum trichloride, the dripping temperature of the acetyl chloride is kept between 50 and 60 DEG C during reaction, the temperature is gradually raised to between 90 and 95 DEG C after the dripping is finished, and reflux stirring reaction is performed for about 4 hours to obtain the 2,4-dichloroacetophenone. The method has the advantages that: the m-dichlorobenzene is reclaimed from the solid waste chlorobenzene tar during production, and the reclaimed m-dichlorobenzene is used as the raw material for producing the 2,4-dichloroacetophenone, so by the method, the 2,4-dichloroacetophenone is produced by using the m-dichlorobenzene as the raw material while extra worries of m-dichlorobenzene and o-dichlorobenzene production enterprises are solved, the supply and demand pressure of chlorobenzene markets is relieved and extended products are further developed and utilized.
Owner:JIANGSU LONGCHANG CHEM
Phase transfer catalyzed method for preparation of polyetherimides
Polyether polymers, such as polyetherimides, are prepared by the reaction of a dihydroxy-substituted aromatic hydrocarbon alkali metal salt, such as bisphenol A disodium salt, with a bis(N-(chlorophthalimido))aromatic compound, such as 1,3- and / or 1,4-bis(N-(4-chlorophthalimido))benzene, in a solvent such as o-dichlorobenzene and in the presence of a phase transfer catalyst such as a hexaalkylguanidinium chloride. Several embodiments may be employed to improve the method. They comprise employing substantially dry reagents, employing a high solids level in solvent, beginning with an excess of bis(N-(chlorophthalimido))-aromatic compound and incrementally adding alkali metal salt, employing alkali metal salt of small particle size, and using reagents of high purity.
Owner:SHPP GLOBAL TECH BV
Method of making bisimides
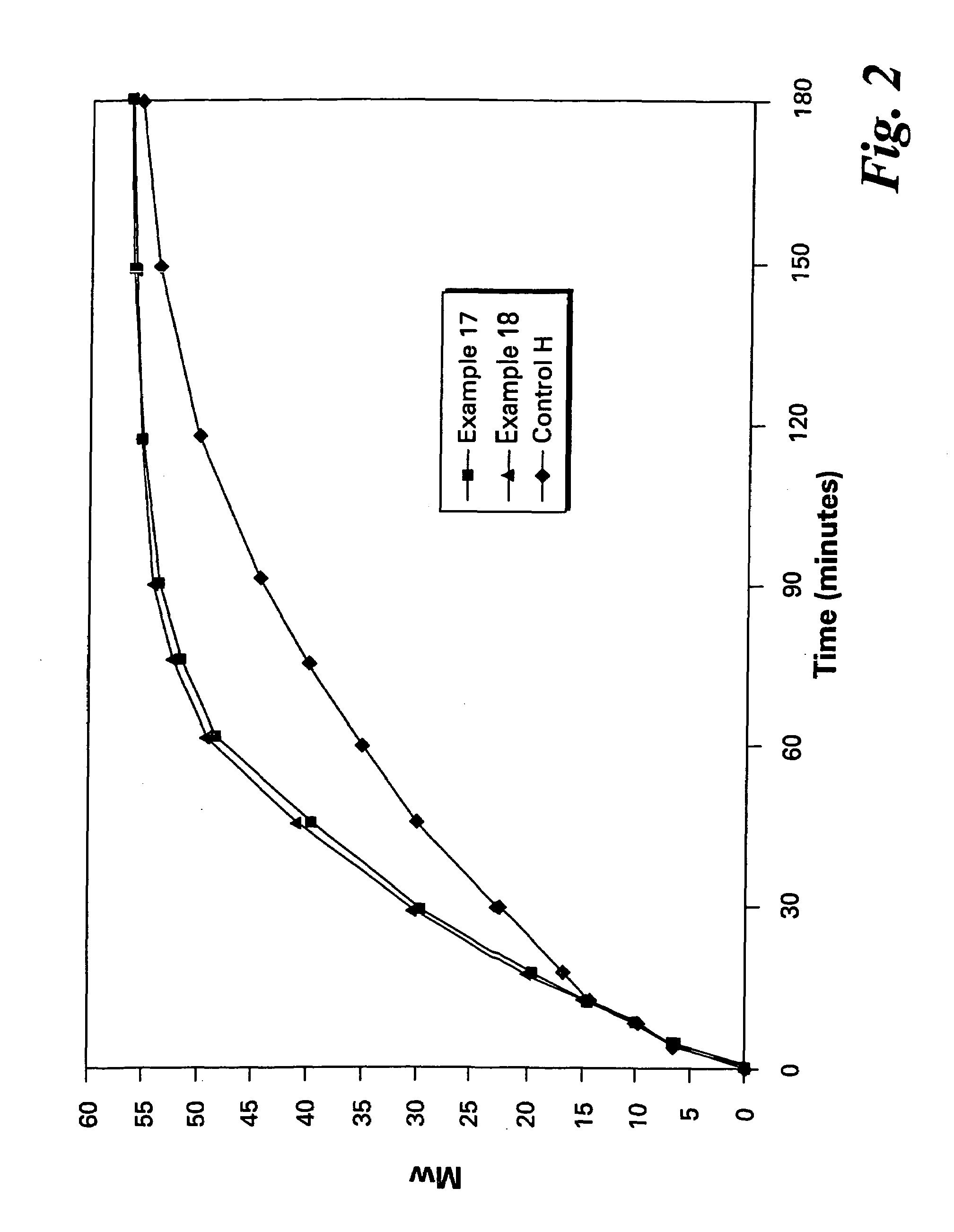
The present invention provides a method for preparing relatively insoluble bisimides under conditions which afford high imidization reaction rates and which permit the monitoring and adjustment of reactant stoichiometry at any stage of the reaction. The bisimides provided by the present invention are prepared either by reaction of a diamine such as 4,4′-diaminodiphenylsulfone (DDS) with an anhydride, for example 3-chlorophthalic anhydride (3-ClPA) in the presence of a solvent at a pressure greater than one atmosphere and at a temperature above the normal boiling point of the solvent, or by reaction of a monoamine with a dianhydride under the same conditions. In one embodiment, the relatively insoluble product bisimides provided by the present invention have a solubility in ortho-dichlorobenzene of less than about 10 percent by weight at a temperature of about 180° C.
Owner:SHPP GLOBAL TECH BV
Method of making bisimides
The present invention provides a method for preparing relatively insoluble bisimides under conditions which afford high imidization reaction rates and which permit the monitoring and adjustment of reactant stoichiometry at any stage of the reaction. The bisimides provided by the present invention are prepared either by reaction of a diamine such as 4,4′-diaminodiphenylsulfone (DDS) with an anhydride, for example 3-chlorophthalic anhydride (3-ClPA) in the presence of a solvent at a pressure greater than one atmosphere and at a temperature above the normal boiling point of the solvent, or by reaction of a monoamine with a dianhydride under the same conditions. In one embodiment, the relatively insoluble product bisimides provided by the present invention have a solubility in ortho-dichlorobenzene of less than about 10 percent by weight at a temperature of about 180° C.
Owner:SHPP GLOBAL TECH BV
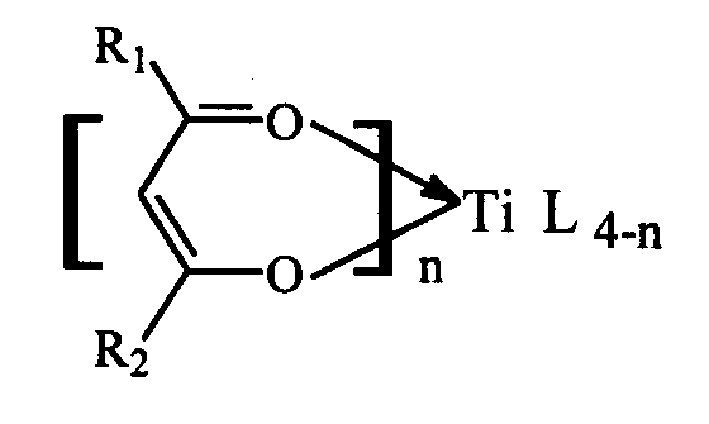
Technical method of preparing polynorbornene using beta diketone titanium non cyclopentadienyl catalyst
A process for preparing polynorbornene with non-cyclopentadiene beta-diketone titanium catalyst includes, adding beta-diketone titanium as primary catalyst, then adding norbornene monomer solution, further adding toluene or o-dichlorobenzene as solvent for dissolving the catalyst, then further adding methyl aloxyane, triethyl aluminium, or triisobutyl aluminium as secondary catalyst, and reaction at 0-90 deg.C. Its product has high-temp. resistance (more than 400 deg.C) and high-vitrification temp. (300-400 deg.C).
Owner:INST OF CHEM CHINESE ACAD OF SCI
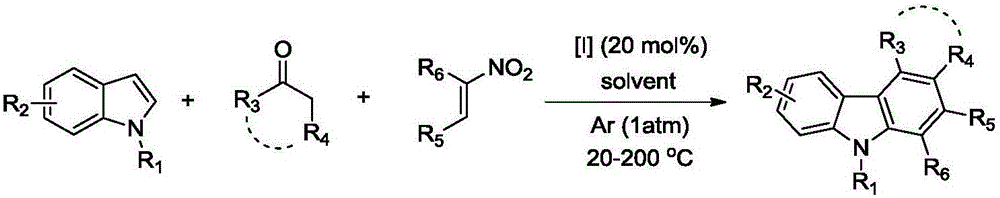
Polysubstituted carbazole, derivative and synthesis method thereof
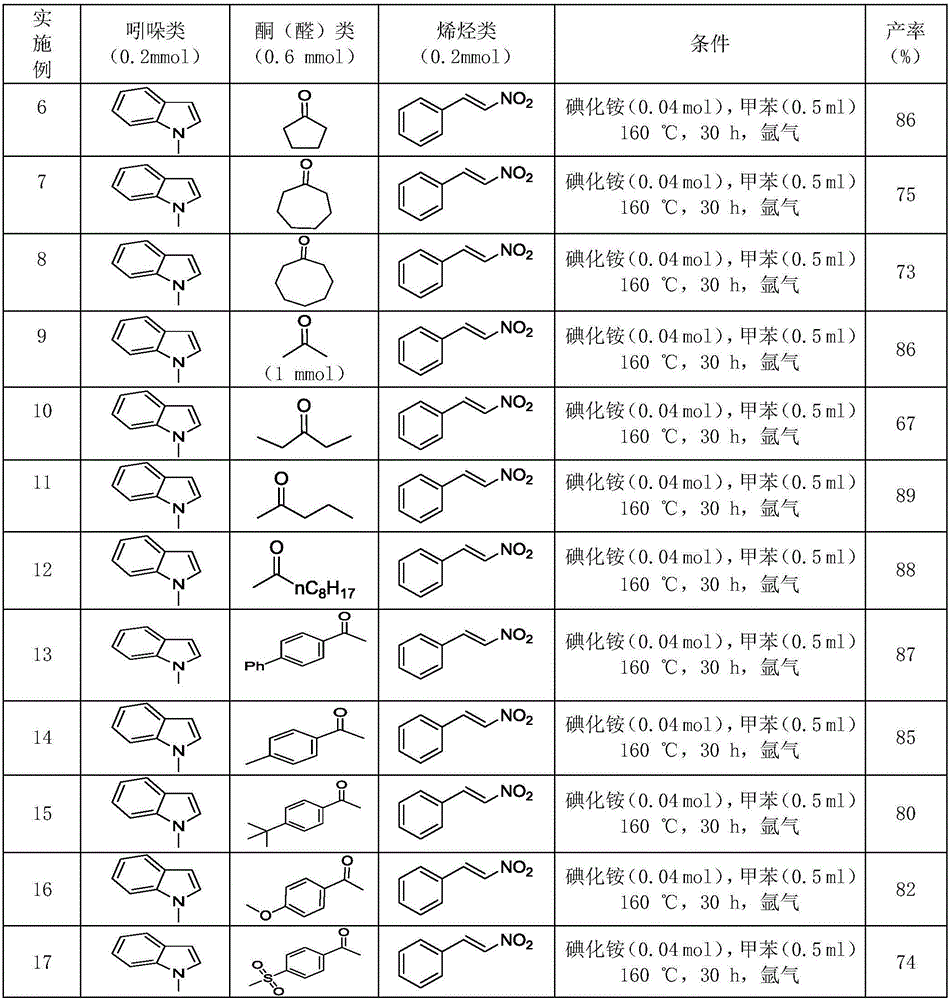
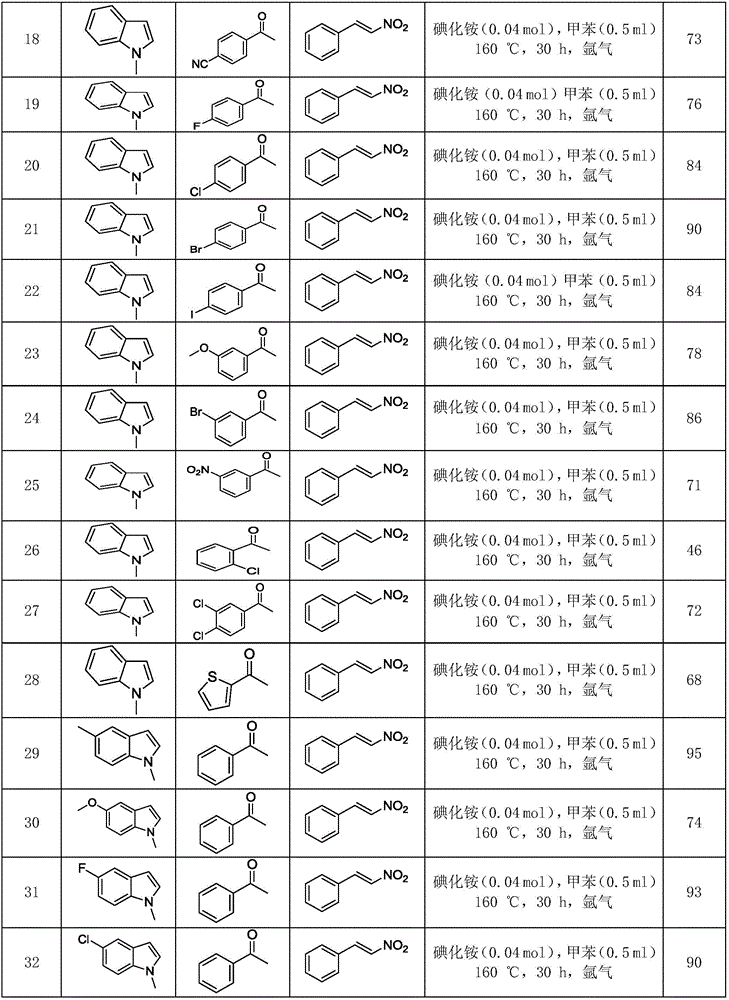
ActiveCN106117113AReduce pollutionStable molecular structureOrganic chemistryKetoneMolecular recognition
The invention discloses polysubstituted carbazole, a derivative and a synthesis method thereof. The technical scheme includes that under inert gas shielding, three simple components including indole, alkene, and ketone (aldehyde) are adopted for selective synthesis of the polysubstituted carbazole and the derivative for the first time by adoption of ammonium iodide as a catalyst for the first time and adoption of acetonitrile, dichloroethane, tetrahydrofuran, N,N-dimethyl acetyl, benzene, chlorobenzene, orthodichlorobenzene, cyclohexane, 1,4-dioxane, anisole, benzonitrile, dimethylbenzene, trifluorotoluene, methylbenzene, trimethylbenzene and the like as organic solvents. The defect that an existing synthesis method is complex in synthesis steps and requires multi-step synthesis, tradition metal catalysts, chemical equivalent metal oxidizing agents and the like is overcome. The polysubstituted carbazole, the derivative and the synthesis method are widely applicable to various fields of photoelectricity, printing and dyeing, medicines, molecular recognition and the like and is especially suitable for research and development of metal-catalysis-free multi-component one-pot selective synthesis of polysubstituted carbazole compounds.
Owner:XIANGTAN UNIV
Aqueous-phase synthesis method of chlorpyrifos by using trichloro-acetic chloride as initial material
InactiveCN102993237ASimple methodEasy to operateGroup 5/15 element organic compoundsFiltrationAcrylonitrile
The invention discloses an aqueous-phase synthesis method of chlorpyrifos by using trichloro-acetic chloride as an initial material. The method comprises steps of: reacting trichloro-acetic chloride, acrylonitrile, orthodichlorobenzene and a compound catalyst to obtain a 3,5,6-Trichloropyridin-2-ol sodium crude product; adding the 3,5,6-Trichloropyridin-2-ol sodium crude product into a reaction kettle with water, a catalyst and alkali, heating for reflux for 3 h, standing to separate out a solvent, and cooling, crystallizing and filtering an aqueous-phase to obtain the 3,5,6-Trichloropyridin-2-ol sodium; and adding water into the reaction kettle, adding a dispersing agent, 3,5,6-Trichloropyridin-2-ol sodium, ethyl chloride and a catalyst with stirring, reacting, cooling, crystallizing, washing and conducting suction filtration to obtain a chlorpyrifos active compound product. The invention employs trichloro-acetic chloride as the initial material to produce high-purity 3,5,6-Trichloropyridin-2-ol sodium, which is used directly as a raw material for the synthesis of chlorpyrifos. The sodium alkoxide produced by the method does not require refining, purifying or drying; water is used as the medium; and reaction materials are added at once for synthesis of chlorpyrifos. The method has advantages of simpleness, easy operation, environmental protection, energy saving, increased product yield, and improved product purity.
Owner:JIANGSU QIHENG AGRI & CHEM TECHCO
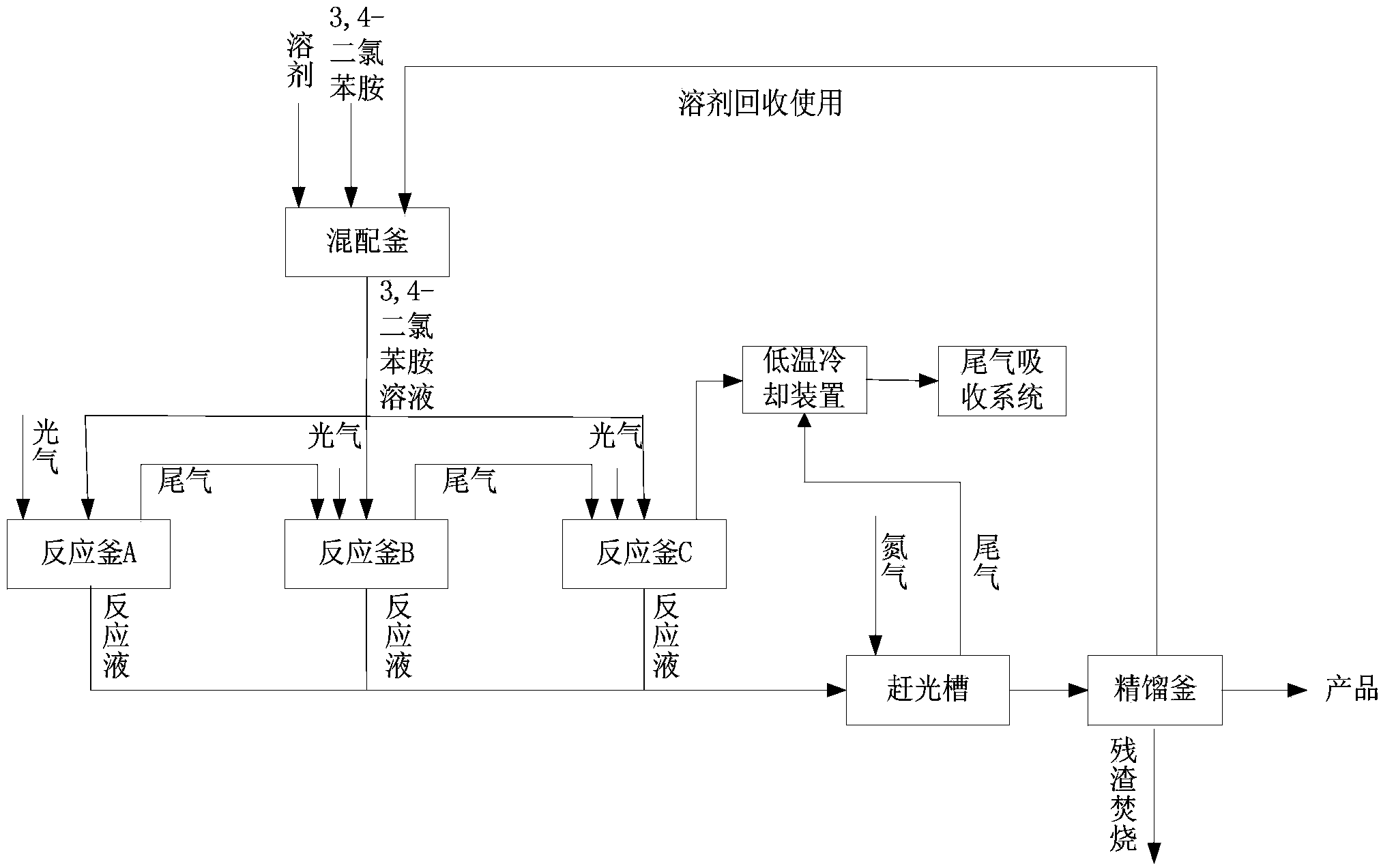
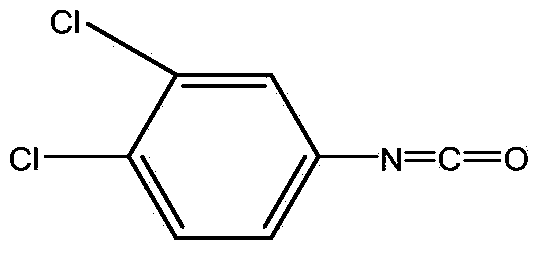
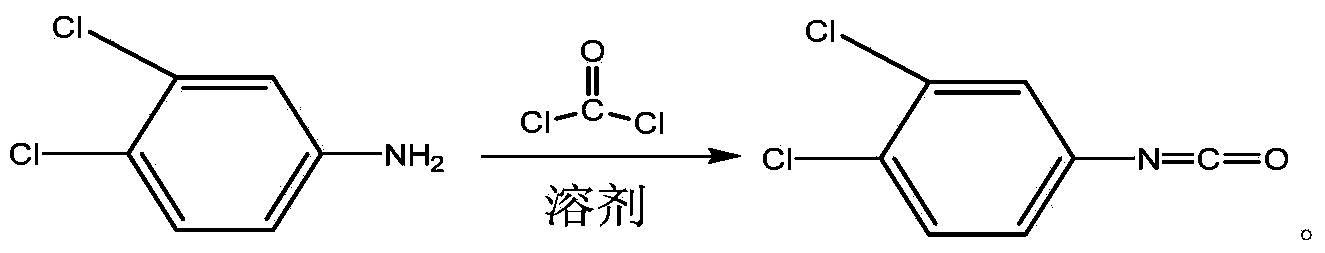
Method for continuously preparing 3,4-dichlorophenyl isocyanate
ActiveCN104356028ATake advantage ofReduce processing energy consumptionIsocyanic acid derivatives preparationOrganic compound preparationChlorobenzeneEthane Dichloride
The invention discloses a method for preparing 3,4-dichlorophenyl isocyanate through continuous feeding reaction by using 3,4-dichloroaniline and phosgene as raw materials and using dichloroethane, methylbenzene, dimethylbenzene, chlorobenzene or o-dichlorobenzene as a reaction solvent. According to the method, hydrochloric acid gas generated in reaction can continuously generate 3,4-dichloroaniline hydrochloride together with 3,4-dichloroaniline, 3,4-dichloroaniline hydrochloride continues to react with phosgene at high temperature to remove hydrochloric acid and generate isocyanate, tail gas containing phosgene and the hydrochloric acid gas generated after reaction of each stage is fully utilized in reaction of next stage, and phosgene is utilized to the maximum extent. The method is safe and environment-friendly; in addition, the energy consumption of the whole process is low and the production efficiency is greatly improved.
Owner:湖南海利常德农药化工有限公司
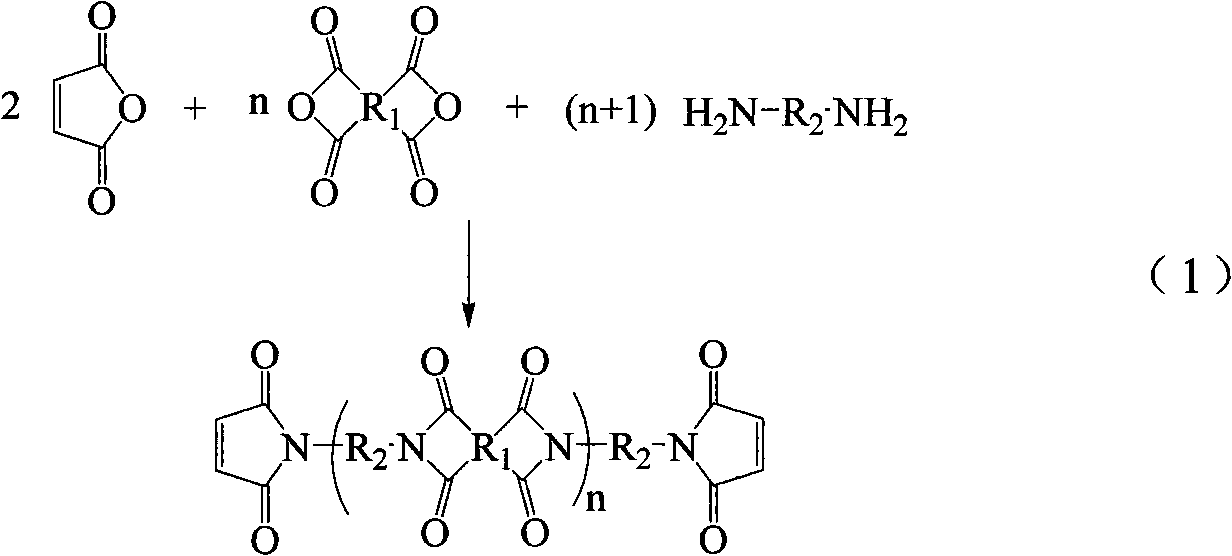
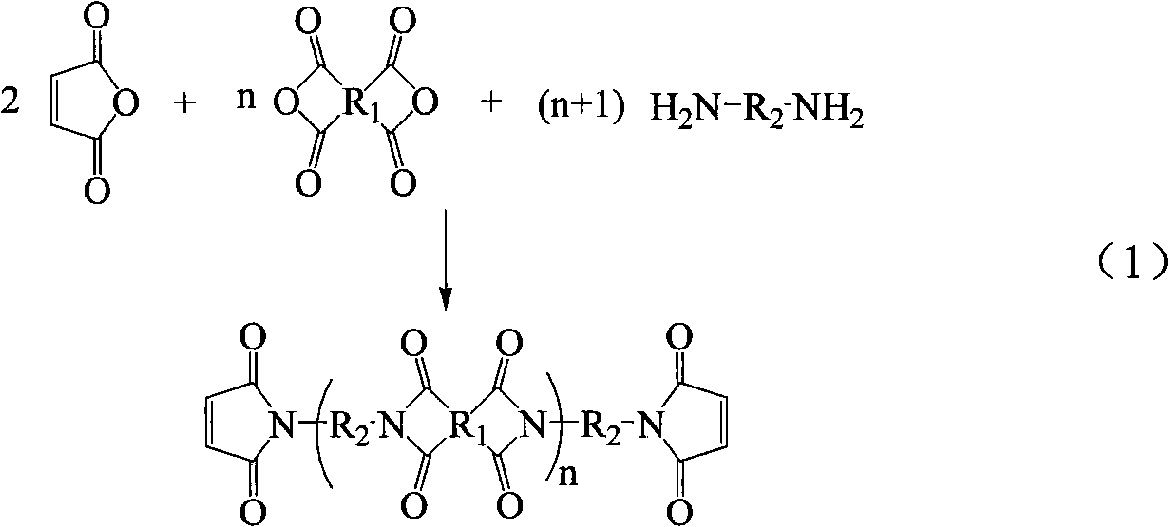
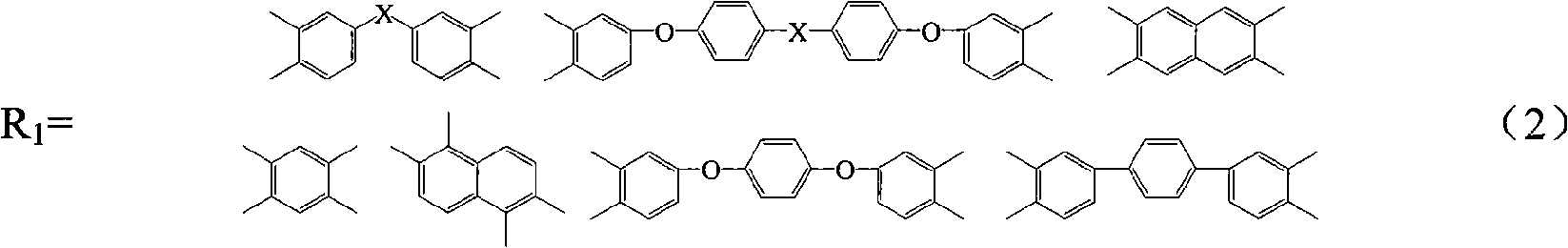
Method for preparing maleimide stop end type polyimide resin
Owner:华烁电子材料(武汉)有限公司
Method for preparing dichlorobenzene and trichlorobenzene and increasing para-ortho ratio
InactiveCN106008143AImprove the contrast ratioGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsHalogenated hydrocarbon preparationBenzeneChlorobenzene
The invention provides a method for preparing dichlorobenzene and trichlorobenzene and increasing the para-ortho ratio, and is characterized in that a chlorination reaction takes benzene or chlorobenzene as a raw material; a continuous chlorination reaction is carried out in the presence of a main catalyst and an auxiliary agent, through a ring type continuous chlorination reactor or a multi-stage series-connection kettle type reactor and under the conditions of the reaction temperature of 50-120 DEG C and the reaction pressure of -0.05-0.5 MPa; and a reaction liquid passes through a membrane filtration system, the main catalyst remains in the reactor for reaction, a dichlorobenzene clear liquid after filtration is subjected to light component removal, rectification and crystallization to obtain dichlorobenzene, orthodichlorobenzene, 1,2,3-trichlorobenzene and 1,2,4-trichlorobenzene finished products. Compared with a conventional dichlorobenzene preparation process, the method has the dichlorobenzene para-ortho ratio greatly increased to 8.5 and the trichlorobenzene para-ortho ratio greatly increased to 13.6, and has the advantages of low use cost of the catalyst auxiliary agent, continuous production realization, good product selectivity and the like.
Owner:JIANGSU YANGNONG CHEM GROUP +2
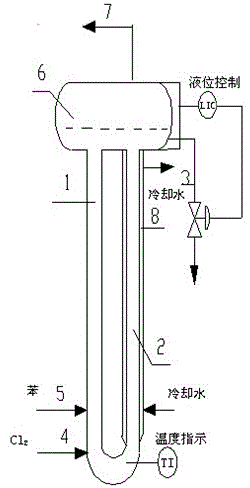
Method and specially used apparatus for ortho-dichlorobenzene continuous mononitration reaction
InactiveCN107417536APrecise control of reaction parametersOvercome problems such as uneven local concentrationProcess control/regulationChemical/physical/physico-chemical stationary reactorsNitrationMetering pump
The invention discloses a method and specially used apparatus for ortho-dichlorobenzene continuous mononitration reaction. The method is characterized in that raw materials are added into an ortho-dichlorobenzene metering tank; a nitrating agent A is added into a nitrating agent A metering tank; the raw materials and the nitrating agent A are separately input into a first mixer synchronously through a first metering pump and a second metering pump; a reaction is performed at a temperature of 100-200 DEG C for 15-50 min; a reaction solution A enters a first liquid separation device; after liquid separation, an organic phase A enters a second mixer and an inorganic phase A enters a waste acid receiving device; a nitrating agent B stored in a nitrating agent B metering tank is added into the second mixer through a third metering pump; a nitration reaction is performed in a second tube reactor at a temperature of 10-100 DEG C; a reaction solution B enters a second liquid separation device; after liquid separation, an inorganic phase B enters the waste acid receiving device and an organic phase B enters a product postprocessing device for obtaining a mononitration product. The concentration of sulfuric acid used in the preparation method provided by the invention is low, the raw material conversion is complete, the yield of the final product is high, multi-nitro by-products are less, and the safety is higher, so that the preparation method and the apparatus are suitable for industrialized production.
Owner:ZHEJIANG UNIV OF TECH
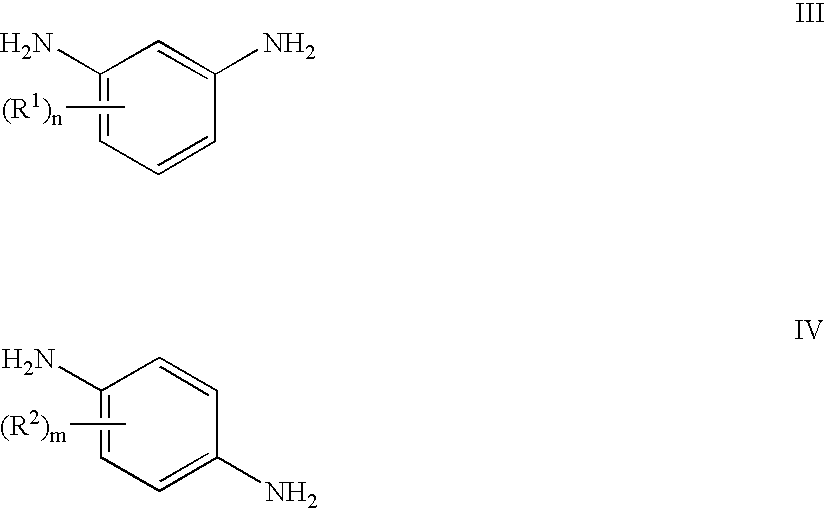
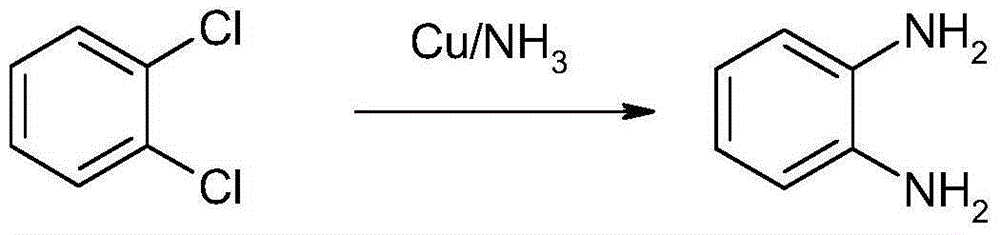
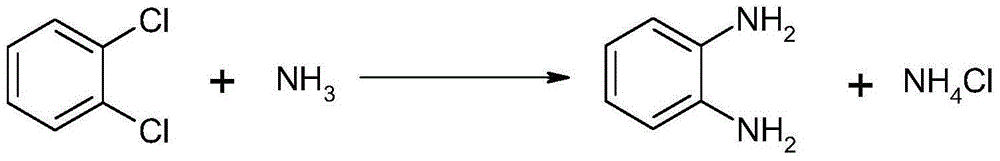
Method for synthesizing and preparing o-phenylenediamine from orthodichlorobenzene
ActiveCN105037171AHigh activityEasy to changeOrganic compound preparationAmino compound preparationReaction temperatureCopper
The invention relates to a method for synthesizing and preparing o-phenylenediamine from orthodichlorobenzene. The method for synthesizing and preparing o-phenylenediamine from orthodichlorobenzene comprises the following steps: putting orthodichlorobenzene, a copper catalyst and a ligand into an autoclave according to a molar ratio of 1:(0.25-5%):(0.25-5%), and then adding liquid ammonia or ammonia water into the autoclave, wherein the molar ratio of the added liquid ammonia or ammonia water to orthodichlorobenzene is (2-20):1; reacting for 2-36 hours while the reaction temperature is controlled to be 20-300 DEG C and the reaction pressure is controlled to be 1-10MPa; and finally, discharging while the inner temperature is controlled to be 90-100 DEG C, transferring into a distillation still, and carrying out vacuum distillation, so that white o-phenylenediamine is obtained. The method for synthesizing and preparing o-phenylenediamine from orthodichlorobenzene has the advantages that orthodichlorobenzene is taken as a raw material, direct amination is carried out to obtain o-phenylenediamine, a high power-supply ligand is adopted for improving the activity of the copper catalyst, the reaction difficulty is reduced, reaction conditions are mild, the reaction efficiency and reaction yield are obviously improved, and thus the method is applicable to industrial production.
Owner:江阴市华亚化工有限公司
Process for extracting high-purity m-dichlorobenzene from solid waste chlorobenzene tar
The invention discloses a process for extracting high-purity m-dichlorobenzene from solid waste chlorobenzene tar, which is a process for extracting the m-dichlorobenzene from a mixture of m-dichlorobenzene, o-dichlorobenzene and p-dichlorobenzene separated from the solid waste chlorobenzene tar by adopting indirect adsorption of zeolite. The process comprises the following steps of: preparing mixed solution of alkanes, then separating a mixture of p-dichlorobenzene and o-dichlorobenzene by using the adsorption of the zeolite, and finally obtaining the m-dichlorobenzene. The process has the advantages that: the process for extracting the m-dichlorobenzene from the isomer mixture is an extraction process implemented by adopting a physical method, and the product obtained by the process hashigh purity and high yield.
Owner:JIANGSU LONGCHANG CHEM
Process for preparing polycrystalline oxotitanium phthalocyanine in mixed crystal regulator
The invention provides a process for preparing polycrystalline form oxytitamium phthalocyanine from the miscible liquidof orthodichlorobenzene and butyl alcohol which consists of the steps of, using orthodichlorobenzene and different volume ratio of butyl alcohol mixed liquid as crystal system modifier, charging purified oxytitamium phthalocyanine, agitating, elevating the temperature to 40-80 deg. C, stirring 4-8h again, cooling down and charging water as precipitating agent, methanol, ethanol or propanol solvent, stirring and filtering, washing the filter cake and drying.
Owner:TIANJIN UNIV
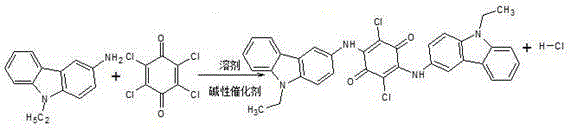
Permanent violet RL intermediate condensation product synthesis process
The present invention discloses a permanent violet RL intermediate condensation product synthesis process, which comprises: alkylating carbazole to prepare N-ethyl carbazole, and carrying out nitrification and reduction to produce 3-amino-N-ethyl carbazole, and carrying out a condensation reaction in an organic solvent to obtain the permanent violet RL intermediate condensate. According t the present invention, the condensation reaction uses the alcohol solvent to replace the conventional o-dichlorobenzene, such that the conversion rate of 3-amino-N-ethyl carbazole is increased, the purity and the yield of the permanent violet RL crude product are improved, the process is stable, the operation is simple, and the safety coefficient is high.
Owner:东台市新锦泰化工有限公司
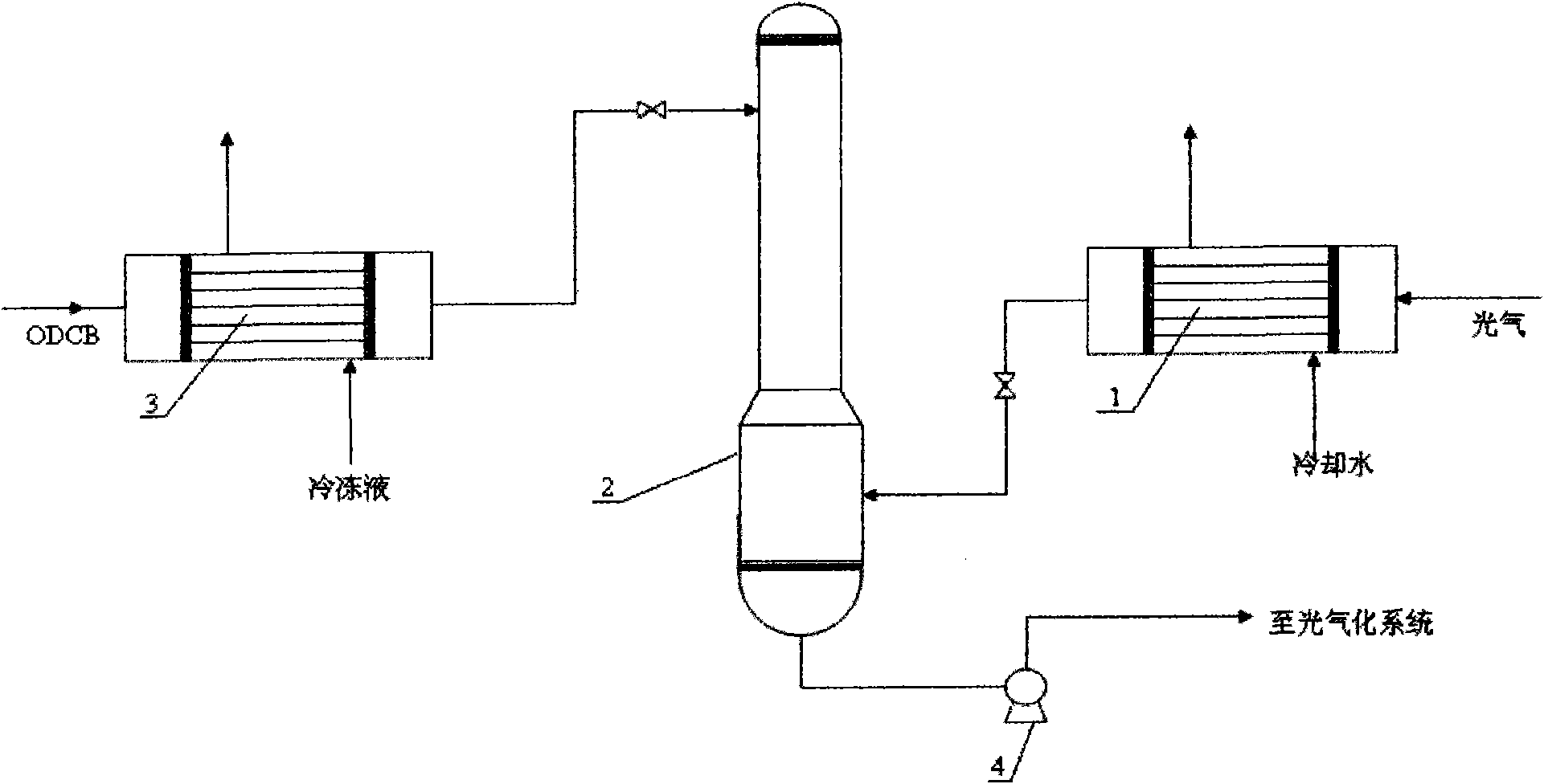
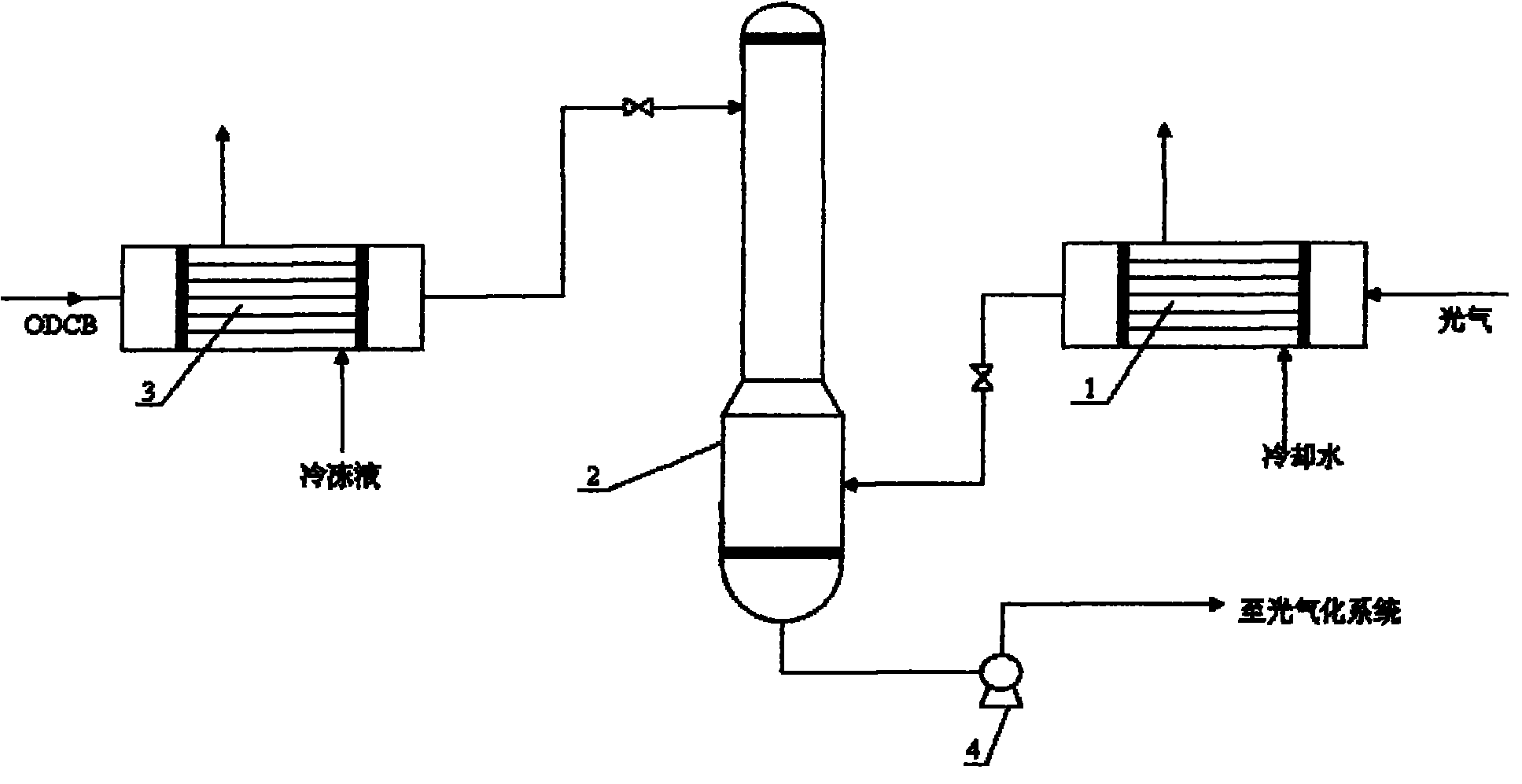
Phosgene treatment method and a method for producing TDI with phosgene
InactiveCN101844999AEasy to operateReduce the difficulty of operationOrganic compound preparationIsocyanic acid derivatives purification/separationPhosgene1,2-Dichlorobenzene
The invention relates to a phosgene treatment method and a method for producing TDI with obtained phosgene. In the production TDI with the phosgene treated by the method, first the temperature of the phosgene GGG is reduced and then the phosgene GGG is added into a phosgene absorption tower, and then low-temperature ODCB is used for absorbing the phosgene; and the treated phosgene is used for producing TDI, so that the operation is more stable.
Owner:蓝星化工有限责任公司
Triethyl phosphite producing process
InactiveCN1887889AWill not polluteProcess conditions are clear and completeGroup 5/15 element organic compoundsDimethylaniline N-oxideTriethylphosphite
The triethyl phosphite producing process with ethanol, phosphorus trichloride, o-dichloro benzene and N, N-dimethyl aniline as material includes the main steps of introducing ammonia, synthesis, water washing, layering, drying and distilling. It has sealed operation process, one distilling step, up reflux system and circular utilization of residual liquid and tail gas, and adopts enamel reactor, conic bottom water washing kettle, serially connected drying tower and other apparatus. The triethyl phosphate is used as plasticizer, stabilizer and additive in producing medicine, pesticide, plastic, dye and lubricant grease. The producing process has high yield, high product purity, no environmental pollution and low production cost.
Owner:泰州市天成化工有限公司
Process for preparing additively structured polynorbornene
The method for preparing polynorbornene by using novel bridged diimine nickel and palladium catalyst includes the following steps: firstly, adding main catalyst, then adding norbornene monomer solution, then adding solvent, finally adding catalyst promoter, the described main catalyst is the bridged diimine nickel and palladium compounding material, its concentration is 1X10 to the minus sixth-1X10 to the minus third mal / L, the described catalyst promoter is methyl aluminium-oxane, triethyl aluminium o r triisobutylaluminium, the mole ratio of the catalyst promotor and main catalyst is 1-10000, the solvent of the described norbornene monomer solution is methylbenzene, and the concentration of monomer in reaction system is 0.01-6.0 mol / L, the solvent of the described system i s methylbenzene, and its reaction temp. is 0 deg.C-90 deg.C.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Process for synthesizing o-chloro-anisole
InactiveCN101407452AEasy to getNo question of choiceEther preparationSodium methoxideSubstitution reaction
The invention discloses a method for synthesizing o-chloro anisole, wherein, o-dichlorobenzene is used as raw material which has a substitution reaction with sodium methoxide to get o-chloro anisole, with the presence of catalyst. The raw materials of the synthesis method are industrial side products which are easy to get; in addition, the operation is simple, with little pollution; the production cycle is short; the yield coefficient of the product is higher; and the product has good industrialization application prospect.
Owner:ZHEJIANG SCI-TECH UNIV
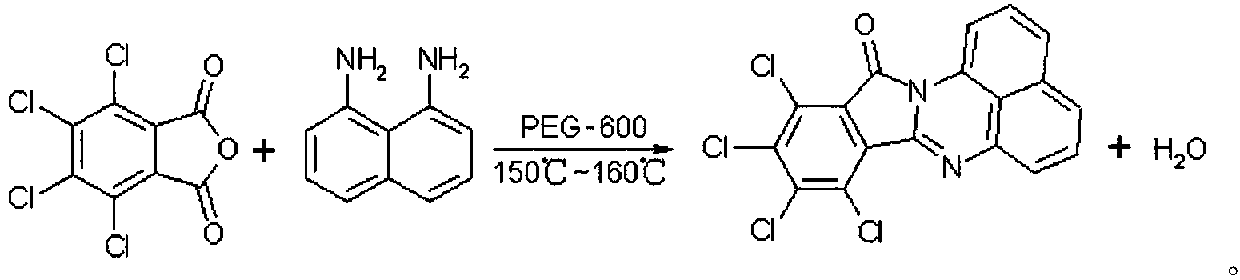
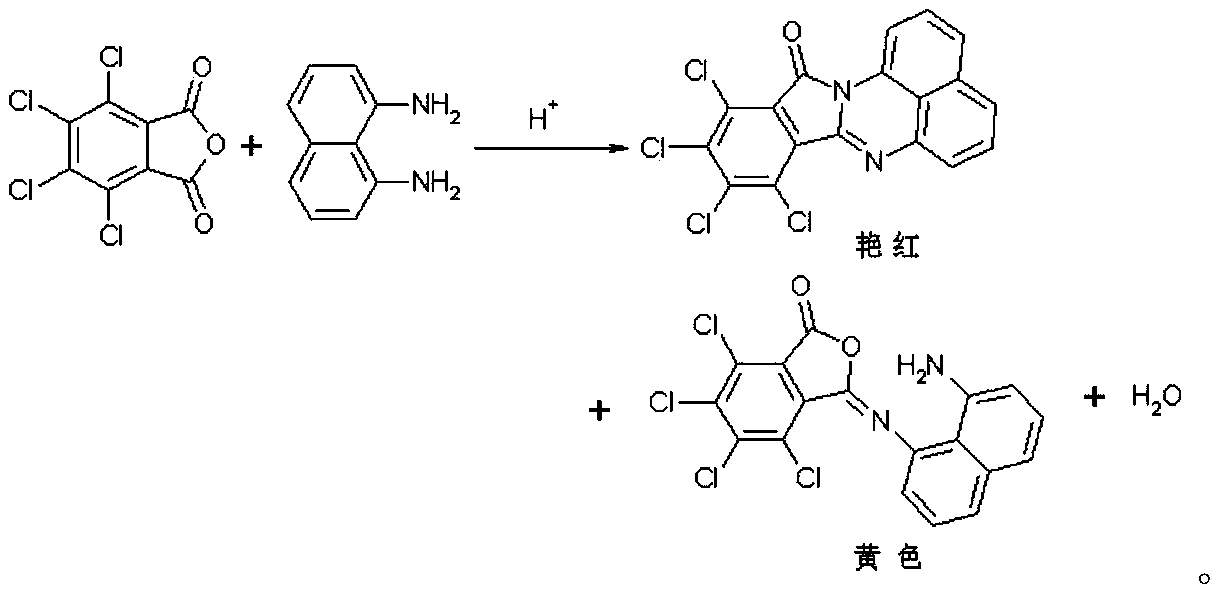
Synthetic method of red naphthalene cyclic ketone dye
The invention discloses a synthetic method of red naphthalene cyclic ketone dye, comprising the steps of with ortho-dichlorobenzene as a reaction solvent, PEG-600 as a catalyst and tetrachlorophthalicanhydride and 1,8-diaminonaphthalene as main materials, holding a certain temperature, isolating water produced in reaction to raise the temperature, performing HPLC (high performance liquid chromatography) medium control, cooling with 1,8-diaminonaphthalene < / =0.5% as a reaction endpoint, separating certain methanol, performing suction filtering, washing filter cake with methanol until there isno smell of ortho-dichlorobenzene, vacuum-drying the filter cake to obtain the target product solvent red 135. The solvent ortho-dichlorobenzene used herein has high boiling point, the recovery rate may reach 95% and above, product yield is high, and the product has good color tone; compared with standards, the product has DC of about 0.5 and weighted strength of about 105.
Owner:JIANGSU DAOBO CHEM
Methods for Preparation of Macrocyclic Polyester Oligomer via Heterogeneous Catalysis
InactiveUS20120302721A1Reduce fermentationImprove efficiencyCatalyst activation/preparationGlass fiberPolyester
The invention relates to methods and systems for preparing macrocyclic polyester oligomer (MPO) directly from monomer via heterogeneous catalysis, rather than by depolymerizing a polyester. For example, in an exemplary embodiment, cyclic poly(butylene terephthalate) (cPBT) is produced by reacting butanediol (BDO) and dimethylterephthalate (DMT) in an organic solvent—for example, ortho-dichlorobenzene (oDCB). The mixture flows over (or otherwise contacts) the catalyst-coated fiberglass or silica gel, e.g., which is packed in a column or bed. MPO is produced in the reaction mixture, while residual linears and catalyst residue remain in the column / bed.
Owner:LIQUID THERMO PLASTICS
Method for preparing 3,5-dibromine-4-hydroxy benzaldehyde
InactiveCN101250097AIncrease profitReduce water contentOrganic compound preparationCarbonyl compound preparation by hydrolysisCresolBenzaldehyde
The invention provides a preparation method of 3, 5-dibromo-4-hydroxy benzaldehyde, which uses p-cresol and o-dichlorobenzen as raw materials, processes low-temperature bromination at 32-42DEG C, high-temperature bromination at 145-168DEG C, hydrolysis, dehydration and drying to synthesize 3, 5-dibromo-4-hydroxy benzaldehyde. The inventive method has clear mechanism, simple operation, high product yield and reduced water content, while the HBr gas generated in preparation can be recovered to be reused, thereby reducing production cost, saving energy and protecting environment. The invention is suitable for industrial production.
Owner:SHANDONG MORIS TECH
Preparation technology of solvent dye
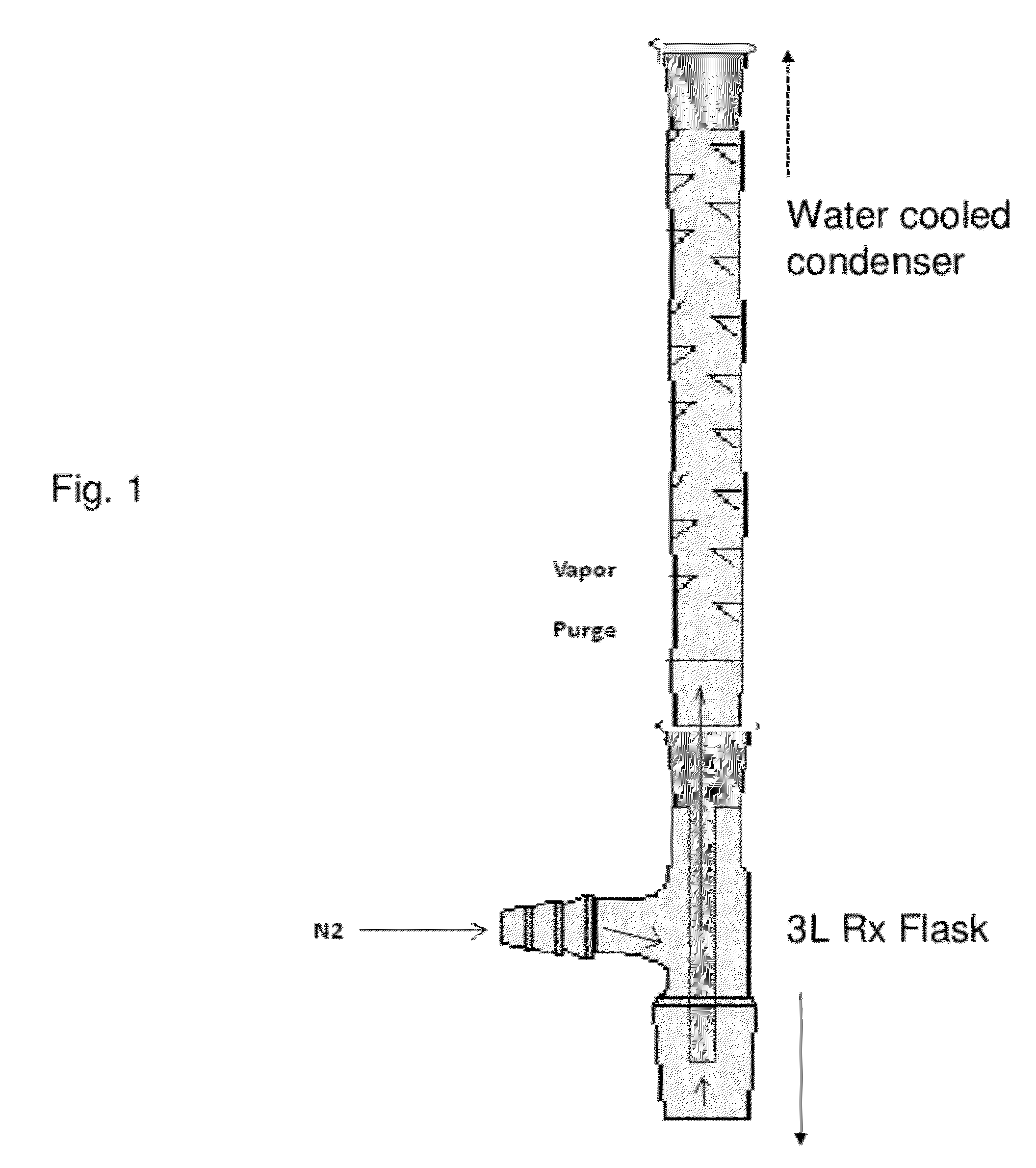
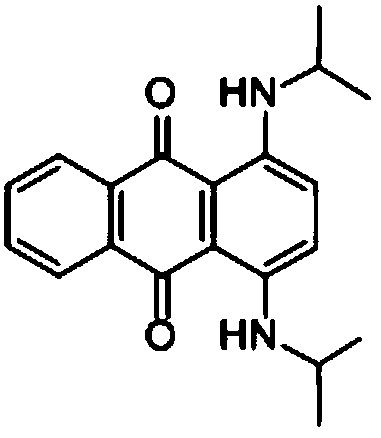
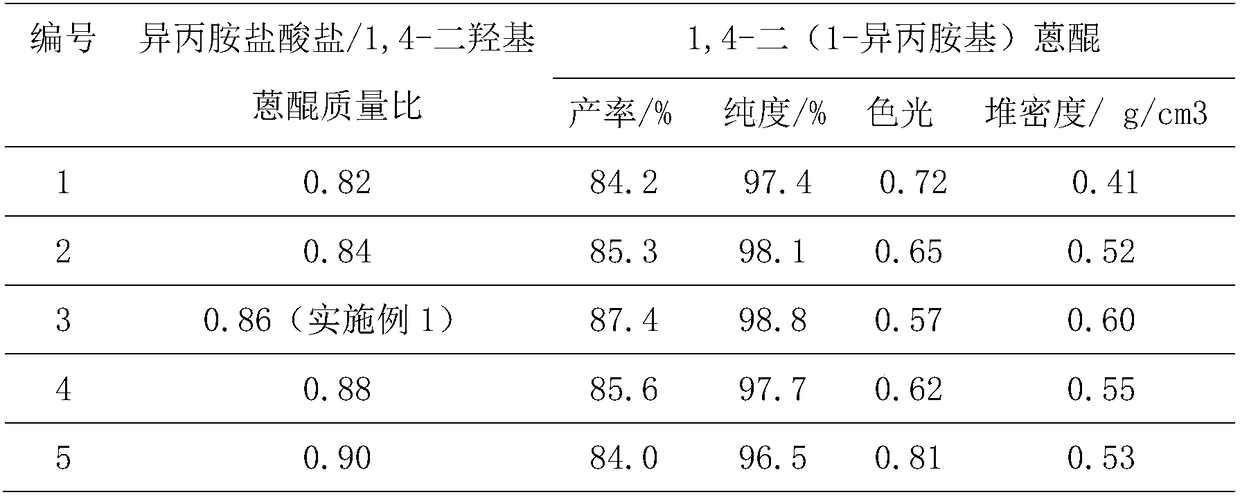
InactiveCN108587224ANo need to recycleReduce processing difficultyAnthracene dyesHigh concentrationIsopropylamine
The invention discloses a preparation technology of a solvent dye. O-dichlorobenzene is taken as a solvent, stannous chloride is taken as a catalyst, and condensation reaction is carried out on 1,4-dioxyanthraquinone and isopropylamine hydrochloride to prepare a target product. According to the preparation technology of the solvent dye, volatile solution isopropylamine is replaced with isopropylamine hydrochloride, the isopropylamine does not need to recycled, high-concentration nitrogen-containing wastewater cannot be generated, thereby reducing the difficulty of wastewater treatment; compared with an aqueous phase method, a solvent method is adopted to prepare 1,4-bi(1-isopropylamido) anthraquinone, and the wastewater quantity is sharply reduced; recrystallization is carried out on a mixed solvent of ethyl alcohol and petroleum ether, a sample of which the purity is 99.0% and the colored light is DC 0.5-1.5 specification can be prepared by adjusting a crystallization solvent, and thepreparation technology has a potential commercial prospect; the product yield can reach 92% or above, and the bulk density is 0.5 gram per cubic centimeter or above.
Owner:JIANGSU DAOBO CHEM
Method for one-step synthesis of adiponitrile from adipaldehyde and ionic liquid type hydroxylamine salt
ActiveCN110467542AThe reaction route is simpleReduce energy consumptionCarboxylic acid nitrile preparationOrganic compound preparationHydroxylamineOrganic solvent
The invention provides a method for one-step synthesis of adiponitrile from adipaldehyde and an ionic liquid type hydroxylamine salt. The method comprises the following steps: adding adipaldehyde, theionic liquid type hydroxylamine salt and an ionic liquid into a reactor, adding an organic solvent, carrying out stirring, then carrying out reflux condensation, and performing a reaction at a temperature of 50-90 DEG C under a normal pressure for 20-100 min to obtain the product adiponitrile, wherein the ionic liquid type hydroxylamine salt is an ionic liquid type hydroxylamine salt of 1-sulfobutyl pyridine bisulfate, the ionic liquid is a 1-sulfobutyl pyridine bisulfate ionic liquid, and the organic solvent is bromobenzene, methylbenzene, p-xylene, benzene, ethylbenzene, o-dichlorobenzene or acetonitrile. The method of the invention realizes one-step clean synthesis of adiponitrile.
Owner:HEBEI UNIV OF TECH
Synthesis process of 1,4-diamino-2-anthraquinone sulfonate
InactiveCN108947872AOrganic compound preparationSulfonic acid preparationChlorosulfuric acidOrganic synthesis
The invention discloses a synthesis process of 1,4-diamino-2-anthraquinone sulfonate, belonging to the field of organic synthesis. The process comprises the steps of adding a 1,4-diamino-anthraquinoneoxysome and o-dichlorobenzene into a container, dropwise adding chlorosulfonic acid while holding the temperature at 30-50 DEG C, heating to 140+ / -3 DEG C, holding the temperature for 6+ / -1 hours, cooling to 60-80 DEG C, transferring to water containing sodium carbonate, stirring for a period of time, adjusting the pH to 7.5+ / -0.1, steam distilling the solvent, cooling to 80+ / -5 DEG C, adjustingthe pH to 1+ / -0.2 with sulfuric acid so as to separate out 1,4-diamino-2-anthraquinone sulfonate, and filtering, so as to obtain the 1,4-diamino-2-anthraquinone sulfonate product. The use amount of chlorosulfonic acid in the process is one third of that of an original process, and the amount of byproduct hydrochloric acid is one third of that of the original process, and no sulfur dioxide gas is produced.
Owner:JIANGSU HUAER CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com