Ultrathin porous Ce-Ni-O-S nanosheet, and preparation method and application thereof
A ce-ni-o-s, nanosheet technology, applied in the field of nanometers, can solve the problems of limiting the commercial application of catalysts, high catalyst overpotential, slow kinetics, etc., and achieve excellent OER performance, short time, and low reaction temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
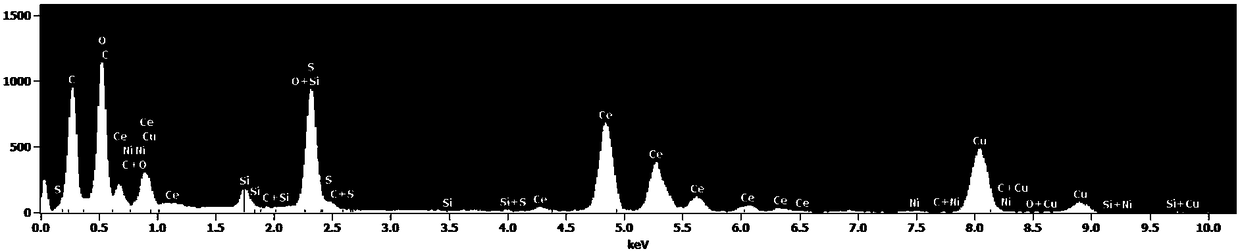
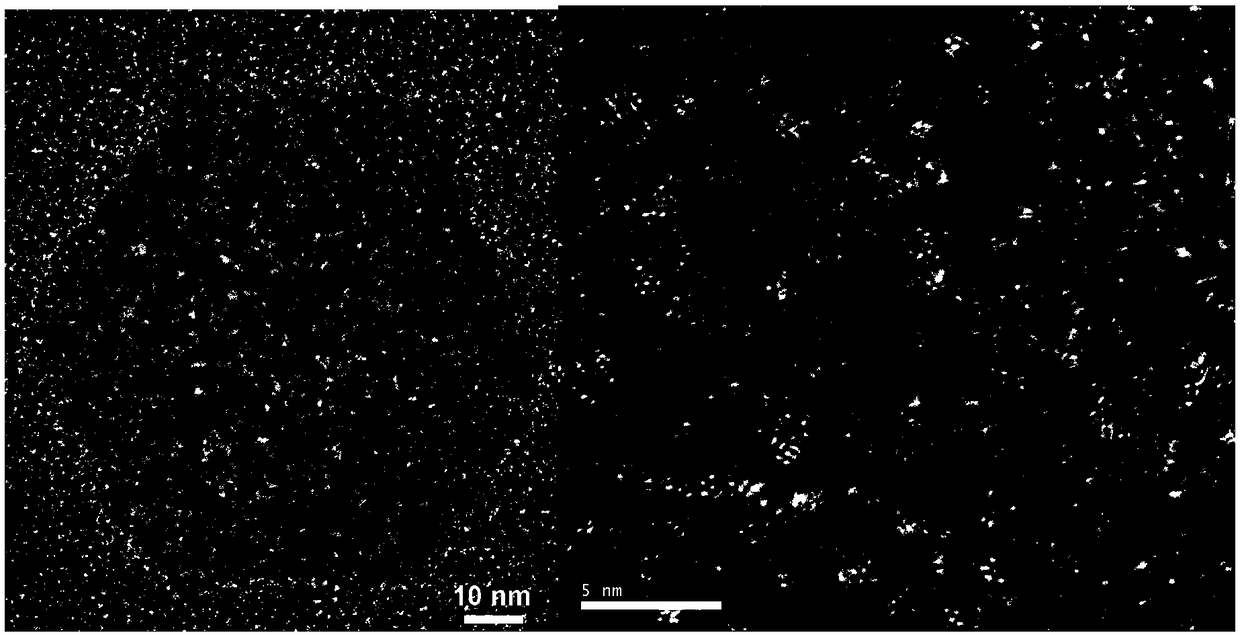
Image
Examples
Embodiment 1
[0029] At room temperature, weigh 55 mg (0.1 mmol) (NH 4 ) 2 Ce(NO 3 ) 6 , 145 mg (0.5 mmol) Ni(NO 3 ) 2 . 6H 2 O, 228 mg (3 mmol) CS(NH 2 ) 2 powder, and all raw materials were added together into a dry three-necked round-bottomed flask with a capacity of 250 mL, and then 5 mL of DDA, 5 mL of ODE, and 3 mL of OA were measured with a graduated cylinder, and added to the three-necked round-bottomed flask, Sonicate and stir until completely dissolved to give a solution.
[0030] The three-neck round bottom flask was transferred to a sand bath, and the temperature was raised to 280 °C at a rate of 8 °C / min under programmed temperature control for 30 min until the reaction was completed. After the reactor was naturally cooled to room temperature, an appropriate amount of n-heptane and ethanol mixed at a volume ratio of 1:1 was added to disperse, and the solid was separated by centrifugation. The solid was washed to obtain a black product, which was used for analysis and...
Embodiment 2
[0034] At room temperature, weigh 55 mg (0.1 mmol) (NH 4 ) 2 Ce(NO 3 ) 6 , 145 mg (0.5 mmol) Ni(NO 3 ) 2 . 6H 2 O, 228 mg (3 mmol) CS(NH 2 ) 2 powder, and all raw materials were added together into a dry three-necked round-bottomed flask with a capacity of 250 mL, and then 5 mL of DDA, 5 mL of ODE, and 3 mL of OA were measured with a graduated cylinder, and added to the three-necked round-bottomed flask, Sonicate and stir until completely dissolved to give a solution.
[0035] The three-necked round-bottom flask was transferred to a sand bath, and the temperature was raised to 280 °C at a rate of 4 °C / min under programmed temperature control for 30 min until the end of the reaction. After the reactor was naturally cooled to room temperature, an appropriate amount of n-heptane and ethanol mixed at a volume ratio of 1:1 was added to disperse, and the solid was separated by centrifugation. The solid was washed to give a black product, which was dried overnight in a vac...
Embodiment 3
[0037] At room temperature, weigh 55 mg (0.1 mmol) (NH 4 ) 2 Ce(NO 3 ) 6 , 145 mg (0.5 mmol) Ni(NO 3 ) 2 . 6H 2 O, 228 mg (3 mmol) CS(NH 2 ) 2 powder, and all raw materials were added together into a dry three-necked round-bottomed flask with a capacity of 250 mL, and then 5 mL of DDA, 5 mL of ODE, and 3 mL of OA were measured with a graduated cylinder, and added to the three-necked round-bottomed flask, Sonicate and stir until completely dissolved to give a solution.
[0038] The three-neck round bottom flask was transferred to a sand bath, and the temperature was directly raised to 280 °C for 30 min until the reaction was completed. After the reactor was naturally cooled to room temperature, an appropriate amount of n-heptane and ethanol were added to disperse, and the solid was separated by centrifugation. The solid was washed to give a black product, which was dried overnight in a vacuum oven.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com