Method for evaluating oxidizing and reducing activity of electrocatalysis material through optimized electrochemical method
A technology of electrocatalytic materials and electrochemical methods, which is applied in the field of evaluating the redox activity of electrocatalytic materials and preparing electrodes, can solve the problems of no symmetry, poor reversibility of electroactive substances, and differences, and achieve the effect of efficient evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
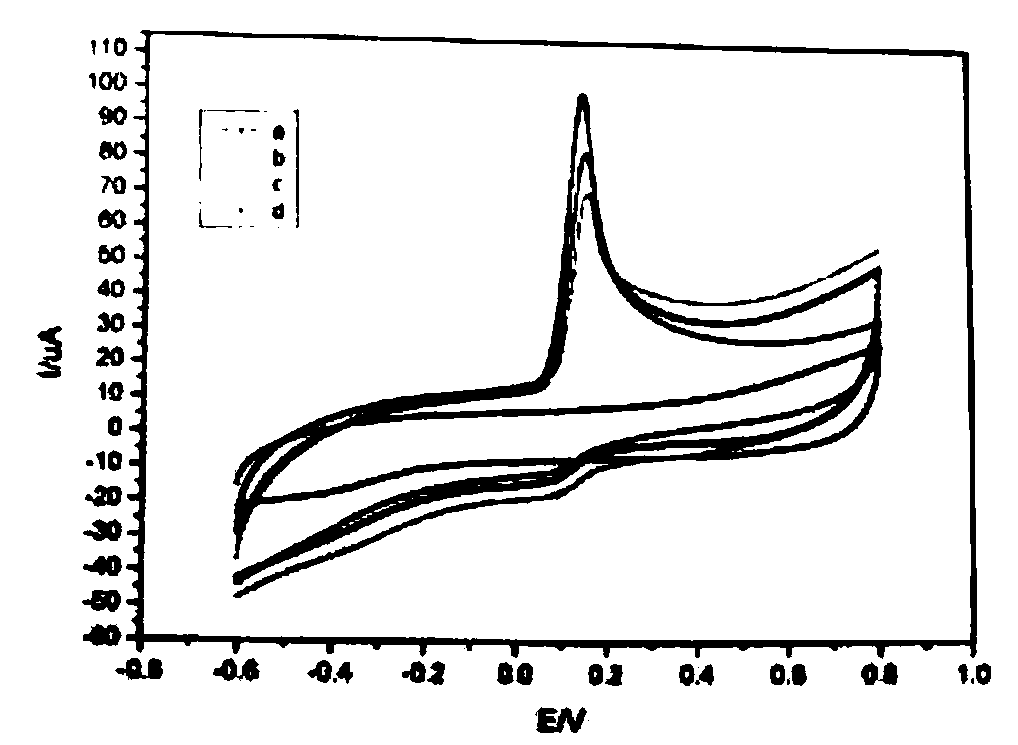
[0015] Comparison of bare glassy carbon electrode, nickel hydroxide nanomaterial modified electrode, nickel hydroxide nanomaterial-graphene modified electrode:
[0016] The bare glassy carbon electrode, the nickel hydroxide nanomaterial modified electrode, and the nickel hydroxide nanomaterial-graphene modified electrode are respectively used as working electrodes, and then connected to the three-electrode system where it is located and the constant potential analyzer, and then the three electrodes are placed Put it into a beaker filled with a certain concentration of ascorbic acid solution (dipotassium hydrogen phosphate as buffer solution, pH adjusted to 4.0), and then measure its CV oxidation curve. The experimental conditions of cyclic voltammetry are: scanning potential range -0.6V~0.8V , Scanning speed 0.10V / S, sampling interval 1mV, static time 1S, cycle 1 time, sensitivity 100μA / V. The cyclic voltammetry curves of different thin film modified electrodes were measured. ...
Embodiment 2
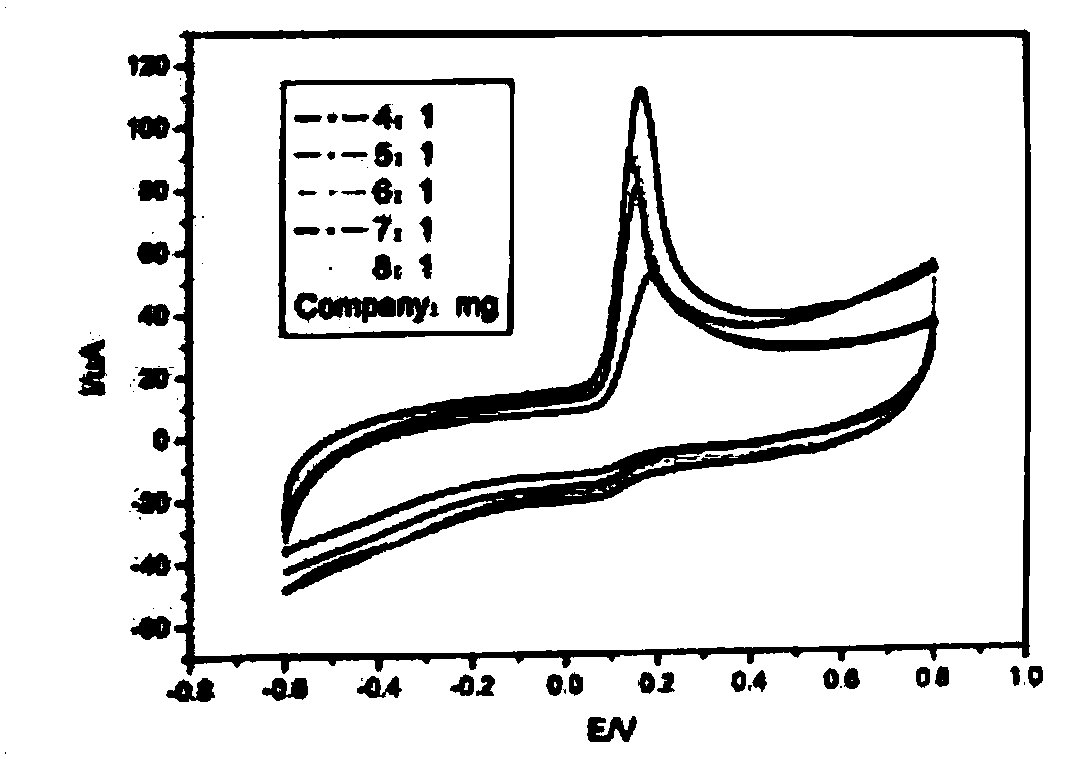
[0022] Comparison of bare glassy carbon electrode, graphene-modified electrode, and platinum nanomaterial-graphene-modified electrode:
[0023] from image 3 It can be seen that there are three oxidation peaks, from high to low, are the electrocatalytic oxidation of ascorbic acid by graphene composite platinum electrode, graphene and bare glassy carbon electrode. It shows that graphene and graphene-composite platinum have improved oxidative properties of ascorbic acid, combined with Figure 4 According to the scanning electron microscope analysis, platinum nanometers can be evenly dispersed on the graphene sheet, and the effect of ascorbic acid determined by graphene composite platinum is significantly increased, and its oxidation performance of ascorbic acid has been significantly improved, which shows that the compounded platinum nanometers The electrocatalytic oxidation of the particles was objectively evaluated.
Embodiment 3
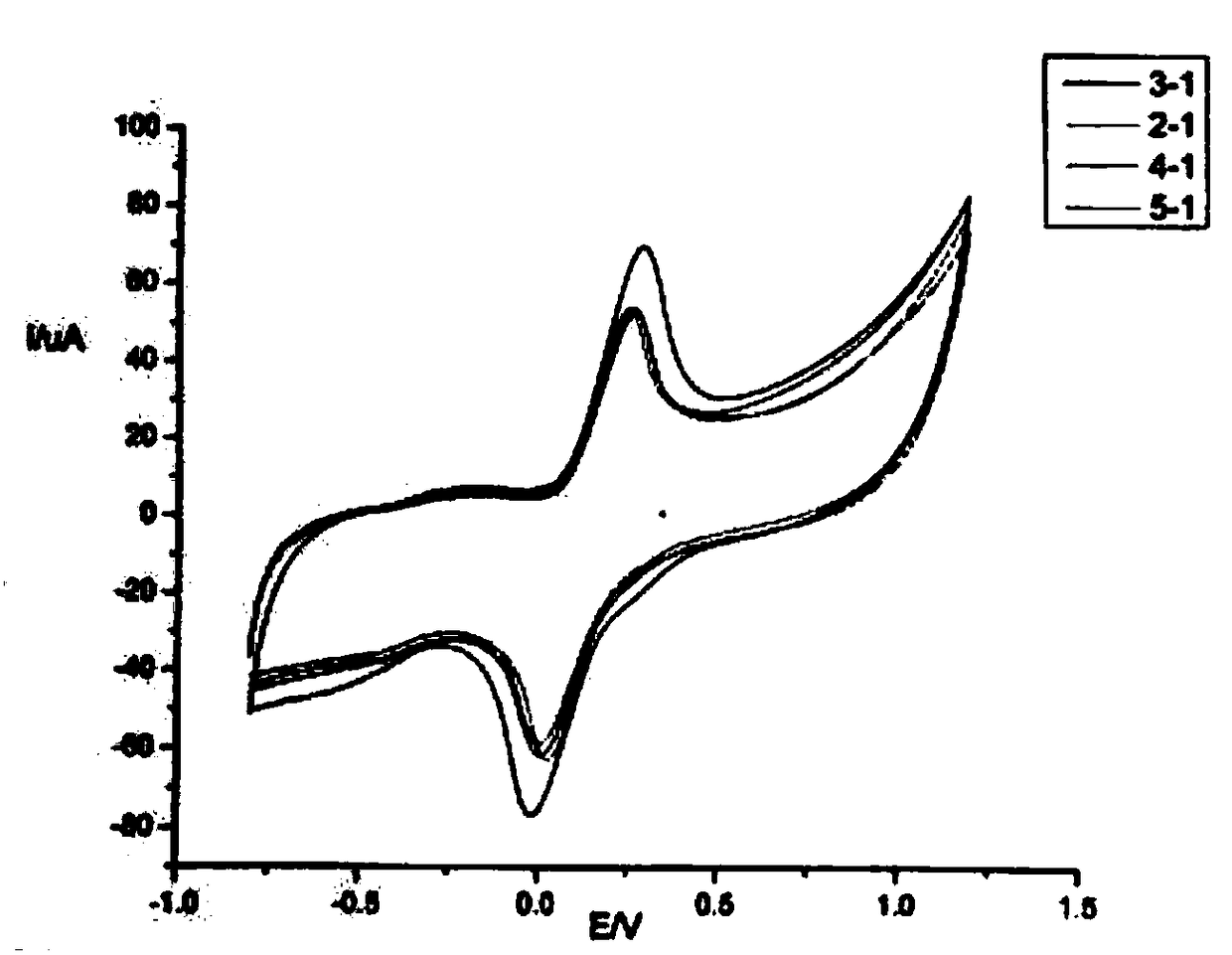
[0025] Comparison of Lanthanum Hydroxide Nanomaterials-Graphene Modified Electrodes:
[0026] The cyclic voltammetry characteristic curve of potassium ferricyanide / potassium ferrocyanide as the probe is modified with different ratio solutions of lanthanum hydroxide-graphene under the same conditions ( Figure 5 ) comparison, it can be concluded that when the ratio of lanthanum hydroxide nanomaterials and graphene increases, the peak current changes significantly; when the ratio of lanthanum hydroxide and graphene is 3:1, potassium ferricyanide / ferrous The peak potential of the potassium cyanide mixed solution is the largest; when the ratio of lanthanum hydroxide and graphene continues to increase, the peak current begins to decrease or almost remains unchanged.
[0027] The cyclic voltammetry characteristic curve of ascorbic acid as a probe was modified with different proportions of lanthanum hydroxide-graphene solution under the same conditions ( Image 6 ) comparative analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com