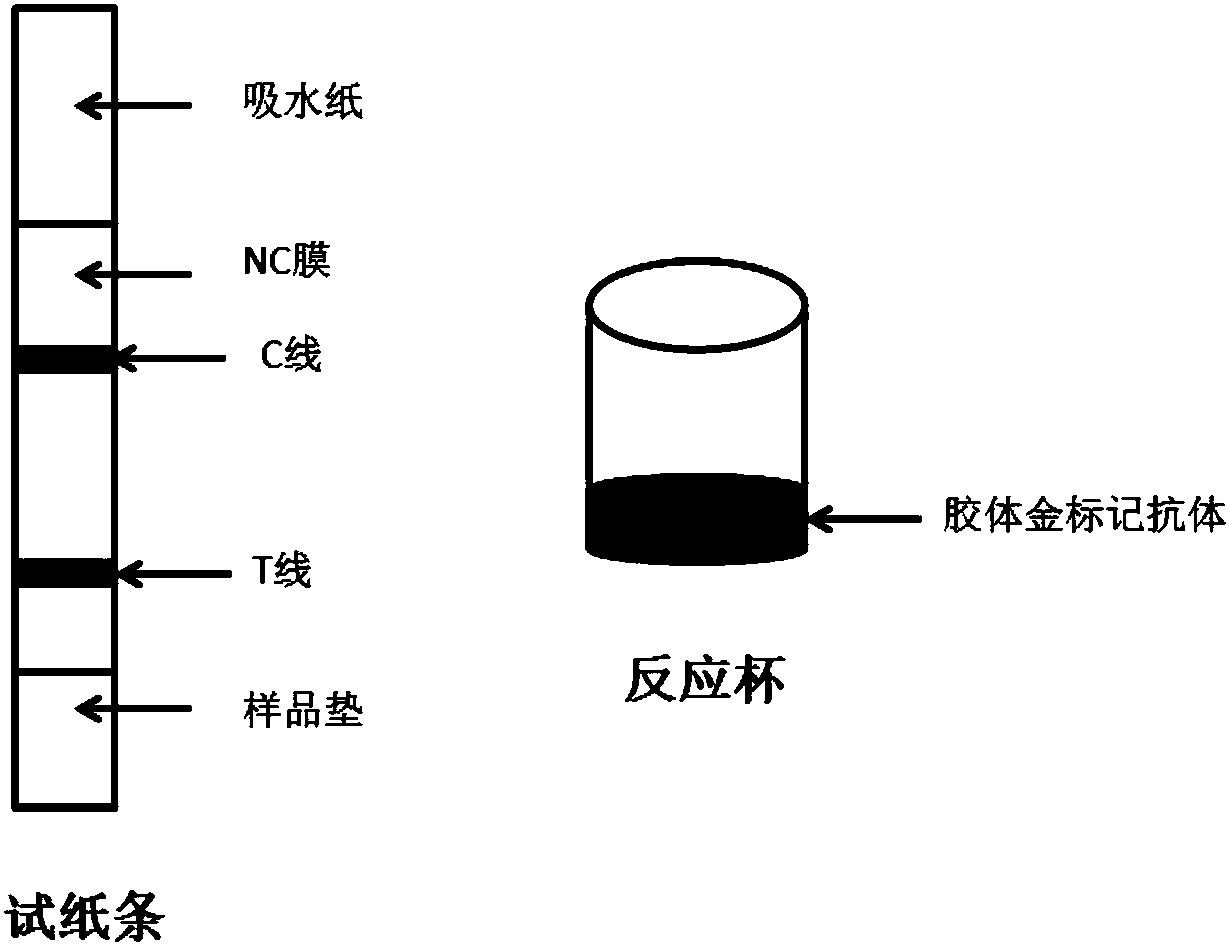
Thiabendazole hapten, coupling antigen and antibody, rapid detection device of colloidal gold and application of rapid detection device
A technology of coupling antigen and detection device, applied in the field of colloidal gold rapid detection device, can solve the problems of high technical requirements of operators, inability to display results immediately, complicated operation of instruments and equipment, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1. Preparation of Thiabendazole Hapten
[0057] The preparation steps of the thiabendazole hapten are as follows: add 2.73g thiabendazole, 1.67g propionbromic acid, and 0.1g sodium carbonate to a 100mL three-neck flask, and react overnight at 70°C in 50mL acetonitrile solution to completely. After the rotary evaporation is completed, the column is extracted with ethyl acetate, so that the thiabendazole hapten can be obtained.
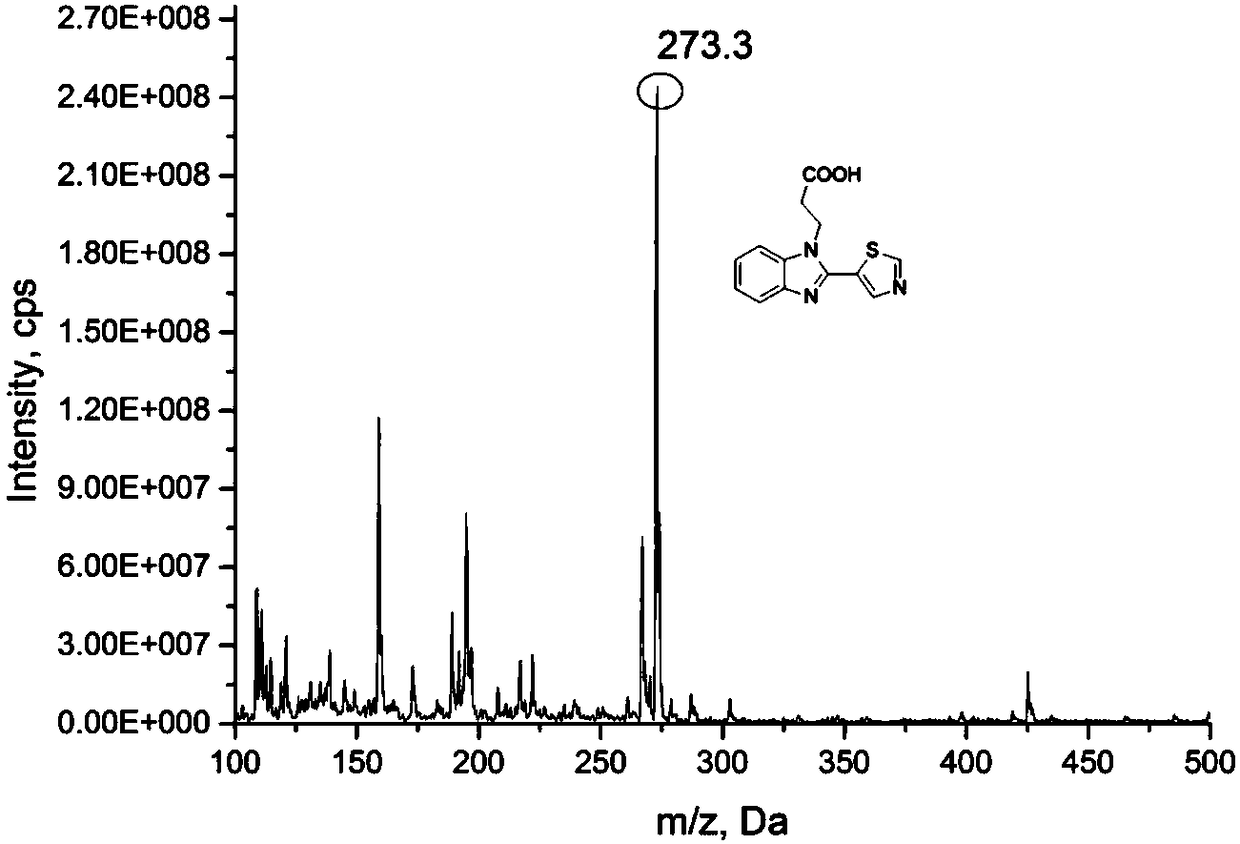
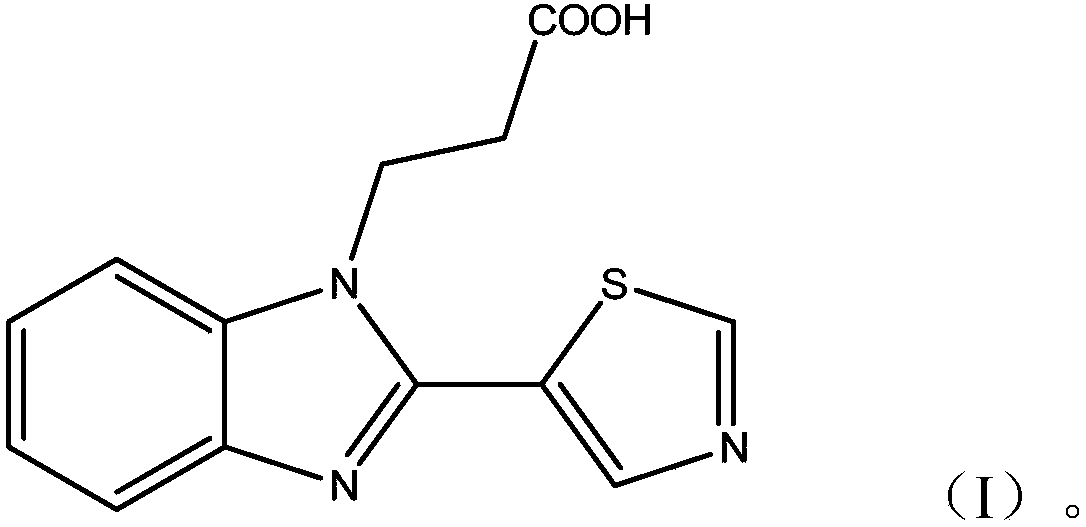
[0058] figure 2 The mass spectrum of the thiabendazole hapten thus obtained is given, and it can be known that the molecular formula of the hapten is as follows:
[0059]
Embodiment 2
[0060] Example 2. Synthesis of Thiabendazole-Coupled Antigen
[0061] Utilize the thiabendazole hapten prepared in Example 1 to prepare the conjugated antigen, specifically as follows: take 0.1 mmol of the thiabendazole hapten and dissolve it in 2 mL of DMF, stir and add 27.5 mg of dicyclohexylcarbodiimide (DCC) and 14.4 mg N-hydroxysuccinimide (NHS). The reaction was stirred overnight at 4°C with magnetic force, and the supernatant was retained overnight after centrifugation, which was labeled as liquid A. Weigh 140 mg of bovine serum albumin (BSA) and hemocyanin (KLH), dissolve them in 10 mL of PBS (pH 8.0) with a concentration of 0.1 mol / L, and then add 1 mL of dimethylformamide (DMF) , stirring and dissolving, thus preparing liquid B. Under magnetic stirring, liquid A was gradually dropped into liquid B, and reacted at 4°C for 12 hours. After centrifugation, the supernatant was taken, and dialyzed with normal saline at 4°C for 3 days, and the dialysate was changed 3 tim...
Embodiment 3
[0062] Example 3. Preparation of Thiabendazole Monoclonal Antibody
[0063] The thiabendazole-conjugated antigen prepared in Example 2 was used to prepare the thiabendazole monoclonal antibody, as follows: four 6-week-old Kunming mice were immunized with the identified thiabendazole-conjugated antigen, and after three booster immunizations, blood was collected Measure the potency. When the serum titer no longer rises, mice are immunized with twice the dose of antigen without adjuvant. Three days later, the mice are killed by decapitation. The spleen is taken under sterile conditions to prepare spleen cells, which are compared with vigorously growing mouse myeloma cells. Mix in a 50mL centrifuge tube at a ratio of 8:1, add 30mL of serum-free IPMI 1640 medium, centrifuge at 1100r / min for 5 minutes, discard the supernatant, shake the cell mass gently, and place in a 37°C water bath. Slowly add 1 mL of 50% PEG-4000 to the cells, drop it within 1 minute, and gently stir the sedime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com