1, 3-diphenyl-1-propanol and method for synthesizing 1, 3-diphenyl-1-propanol from nitromethane
A technology of diphenylpropanol and nitromethane, applied in the direction of organic chemical methods, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of poor practicability and uneconomical splitting methods, and achieve low cost and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
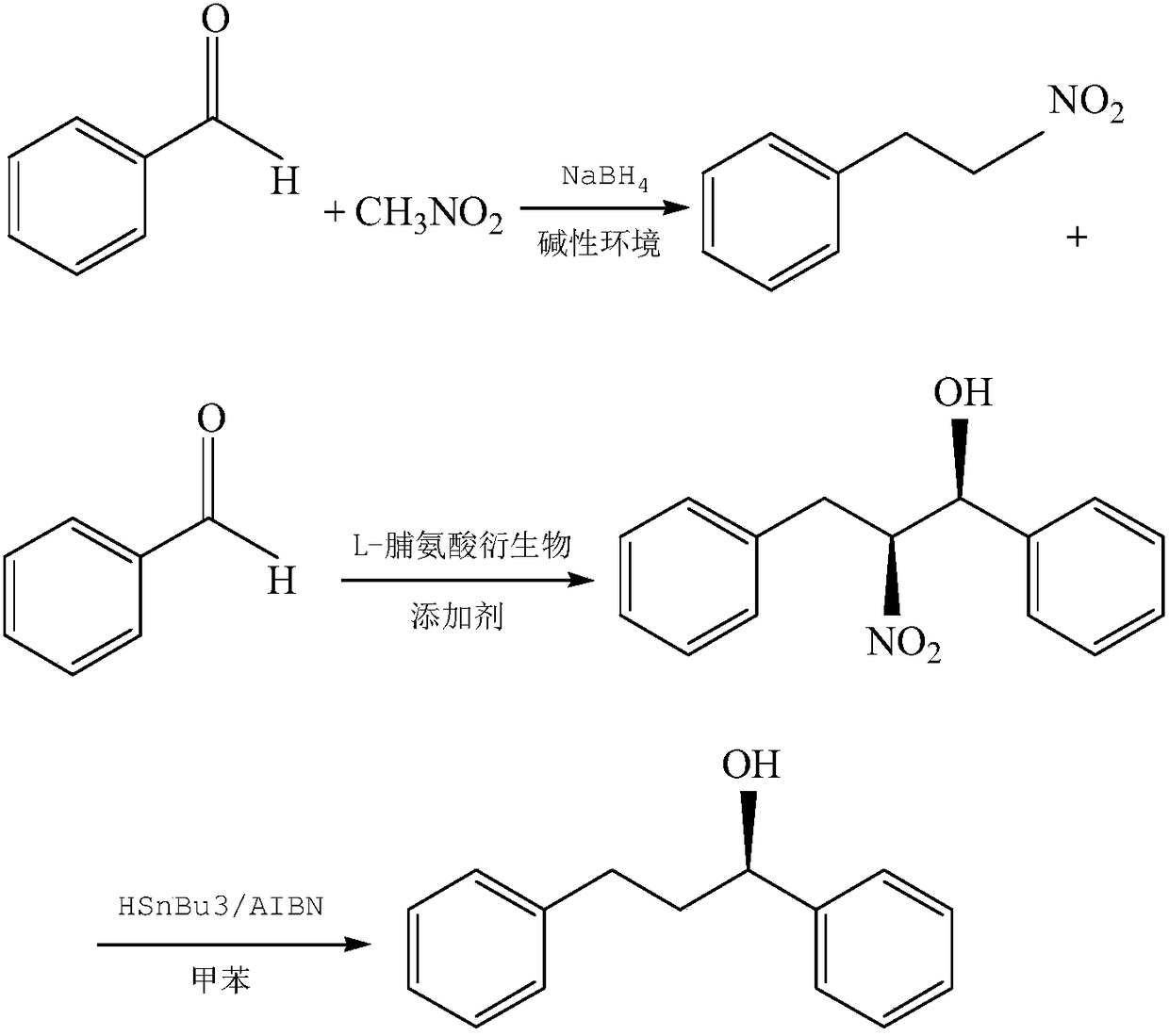
[0070] This embodiment provides a method for synthesizing 1,3-diphenyl-1-propanol from nitromethane, and its specific steps are as follows:
[0071] S11, the preparation of 2-phenylnitroethane, dissolve 6.1g nitromethane (0.1mol), 10.6g benzaldehyde (0.1mol) in 100ml methanol, add 2g sodium hydroxide (0.05mol) under stirring, Stir at room temperature (25°C) until the solution is clear, then add 4g of sodium borohydride (0.11mol), and continue to stir for 2 hours. After the thin plate shows no reactants, acidify with 2N hydrochloric acid, extract with ethyl acetate, and combine the extracts with bicarbonate Wash with sodium aqueous solution and saturated sodium chloride aqueous solution, dry and concentrate to obtain 2-phenylnitroethane.
[0072] S12, the preparation of 3-nitro-1,3-diphenylpropanol, 60mgL-proline derivative (0.25mmol, mole percent 2.5mol%) and 63mg dihydrate cupric chloride (0.25mmol, mole percent 2.5mol%) was added to 40ml tetrahydrofuran, stirred at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com