Isoforrensin A derivative and application thereof to preparation of anti-tumor medicine
A derivative and drug technology, applied in the field of Isoforrensin A derivatives and its application in the preparation of anti-tumor drugs, can solve the problem of narrow anti-cancer spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
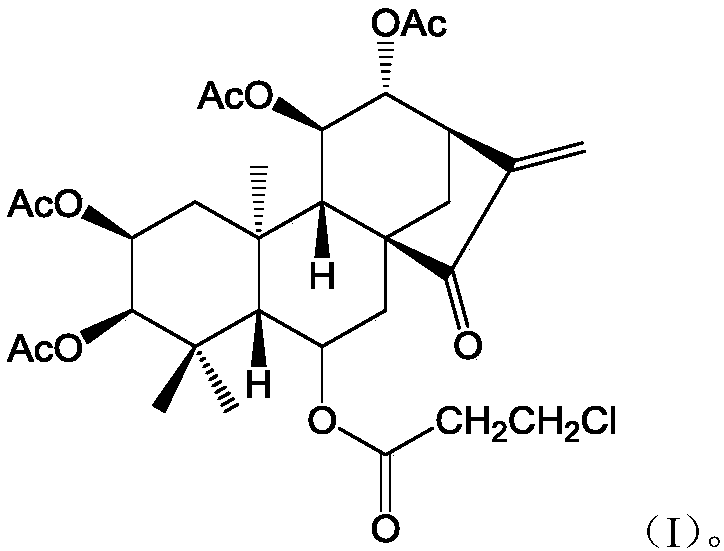
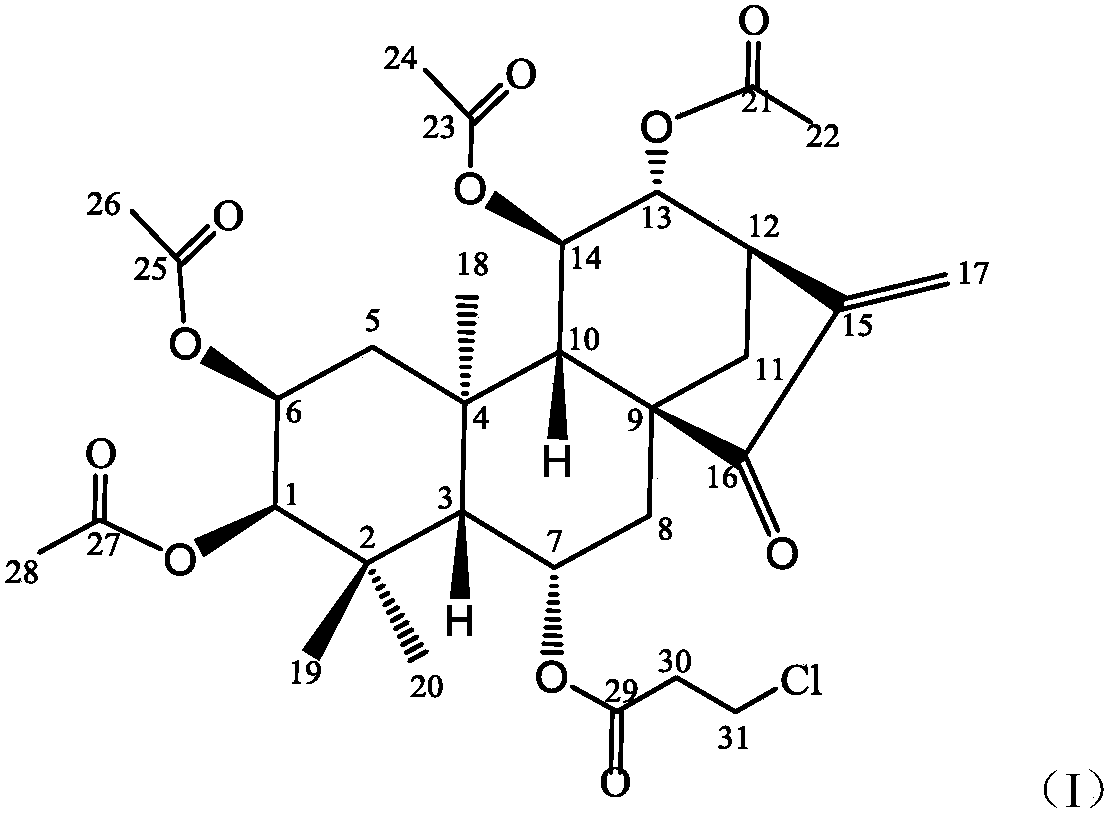
[0015] Synthesis of embodiment 1 formula (I) compound
[0016] 1) Dissolve Isoforretin A and DMAP (4-dimethylaminopyridine) in dichloromethane, add triethylamine, add the corresponding 3-chloropropionyl chloride dropwise at 0°C to 5°C while keeping warm, and heat up the reaction solution after dropping to 20°C to 25°C and stir the reaction.
[0017] 2) After TLC detects that the reaction is complete, add saturated aqueous sodium bicarbonate solution to the reaction solution to quench the reaction, extract the aqueous layer three times with dichloromethane, combine the organic layer, wash the organic layer three times with dilute hydrochloric acid, and wash three layers with saturated saline . The collected organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a crude product. The crude product is purified by flash silica gel column chromatography to obtain pure product target product formula (I) compound;
[0018] The molar ratio of ...
Embodiment 2
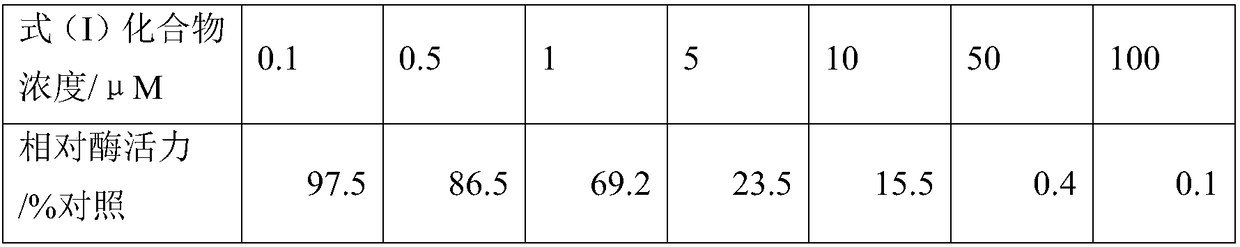
[0038] Pharmacological Example 2 The Inhibitory Effect of Formula (I) Compounds on Different Types of Solid Tumor Cells
[0039] The compound of formula (I) (purity>99%) was dissolved in DMSO, and a 100uM mother solution was prepared and stored at -20°C. Select ten common malignant solid tumors (including lung cancer, gastric cancer, breast cancer, colorectal cancer, liver cancer, cervical cancer, esophageal cancer, oral cancer, prostate cancer, pancreatic cancer), and culture the tumor cell lines of the above ten tumors in vitro and among them The corresponding tissue normal cell lines of the five tumors are as follows:
[0040] Lung cancer: human normal lung epithelial cells HBEC, human lung cancer cells A549, SK-MES-1
[0041] Gastric cancer: human normal gastric mucosal epithelial cells RGM-1, human gastric cancer cells MGC-803, SGC-7901
[0042] Breast cancer: human normal breast epithelial cells MCF10A, human breast cancer cells MCF7, MDA-MB-231
[0043] Colorectal ca...
Embodiment 3
[0053] Pharmacological Example 3, Comparative Test on the Survival Period of Tumor-bearing Mice
[0054] 1. Materials
[0055]a) Cell line: Gastric cancer cell MGC-803, which can stably express luciferase, was purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences.
[0056] b) Animals: 30 BALB / c nude mice, body weight (13±2g), male.
[0057] c) Drug: Iso A (drug 1), formula (I) compound (drug 2)
[0058] 2. Experimental method
[0059] a) Preparation of gastric cancer tumor-bearing mouse model: Gastric cancer cells MGC-803 in the logarithmic growth phase were taken, digested with trypsin and prepared into 1×10 7 cells / mL cell suspension, inoculate 200 μL (100 μl cells + 100 μl Matrigel) cell suspension into the right axilla of nude mice (only 200 μL Matrigel was injected in the normal group).
[0060] b) Grouping and administration of experimental animals: After two weeks of feeding, the tumor-bearing situation in the mice ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com