Application of ligand antrum mucosal protein-18(AMP-18) of laminin receptor in treatment of tumor
A technology of receptors and tumors, applied in the field of bioengineering, can solve problems such as the inability to maintain cell functions, and achieve a good effect of tumor suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
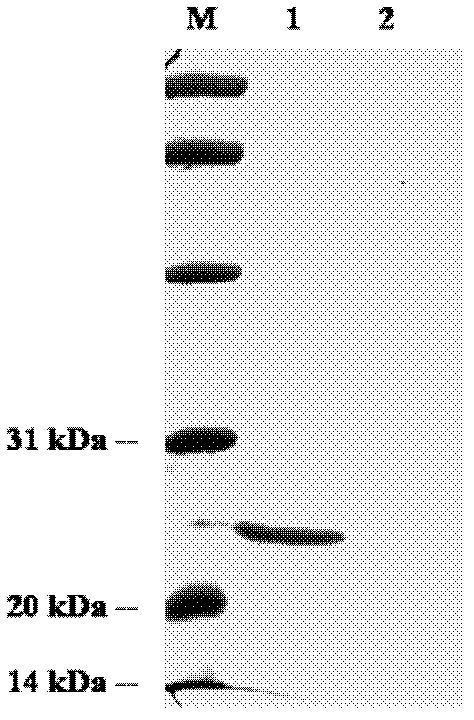
[0033] Example 1 Preparation of AMP-18 recombinant protein
[0034] Using normal gastric tissue cDNA as a template, design specific primers according to the gene sequence of AMP-18 mature peptide (SEQ ID No.2), and introduce BamHI and SalI restriction sites at the 5' ends of the upstream and downstream primers respectively: upstream primer: 5'- TGAAGGATCCAACTATAATATCAACGTC-3'; downstream primer: 5'-AAATGTCGACGTTTCTCCACCGTGTCTCC-3'. The gene encoding the mature peptide sequence of AMP-18 was amplified by PCR, and then double-digested with BamHI and SalI after recovery from the gel. The enzyme-digested fragments were recovered by double gel and ligated with the BamHI / SalI double-digested vector pQE30a overnight at 16°C to chemically transform the competent cell JM109. Pick a single clone to extract the plasmid for double enzyme digestion verification, and sequence the positive clone to verify that the sequence is correct. Inoculate and culture the positive bacteria with correc...
Embodiment 2
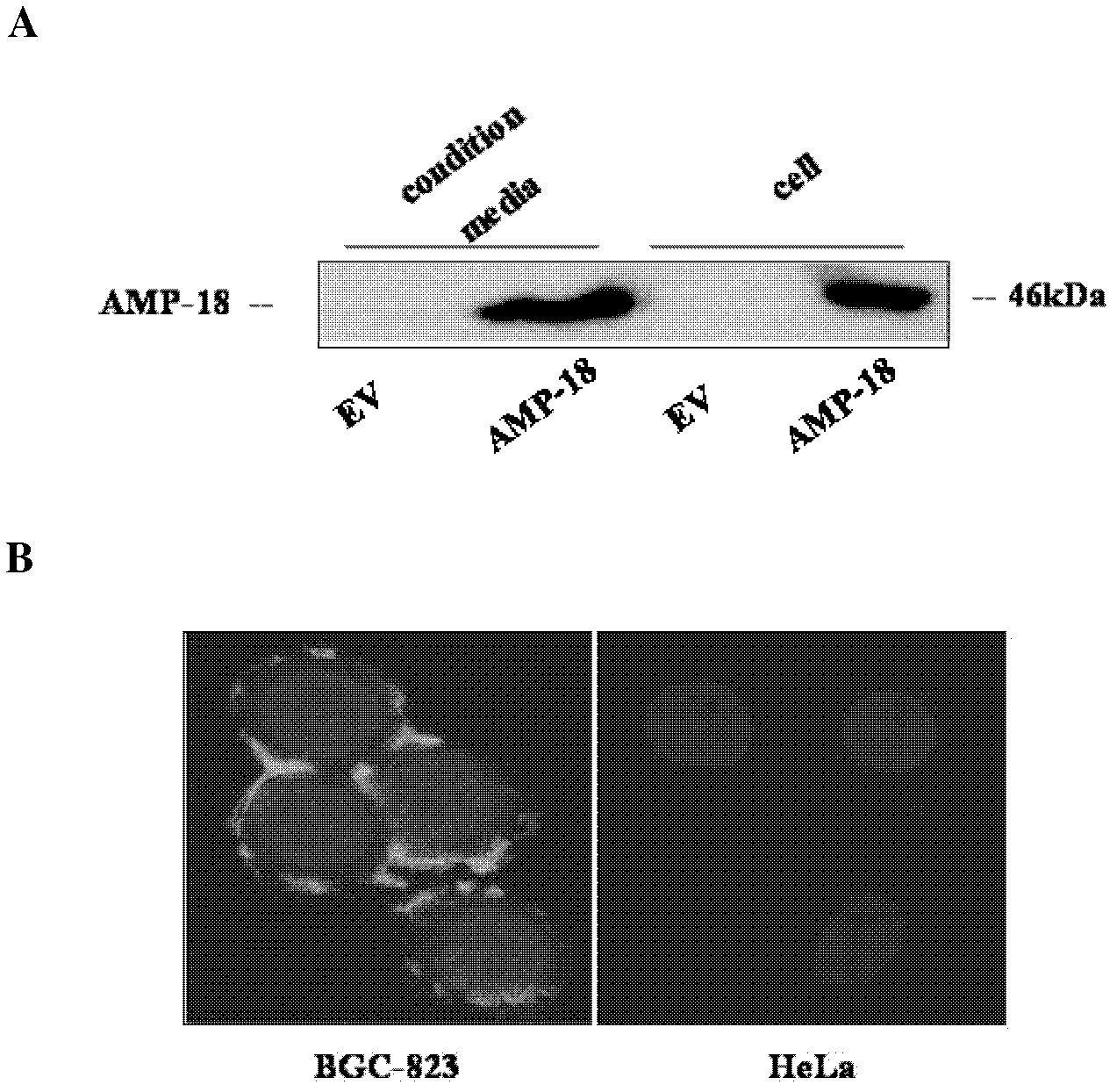
[0036] Example 2 Autocrine and membrane localization of AMP-18
[0037] 1. Western blot detection of AMP-18 expression in cells and culture medium
[0038] The eukaryotic expression plasmid EGFP-AMP-18 cloned on the carrier pEGFP-N1 (Invitrogen Company) and its empty vector EV were transfected into BGC-823 cells by the method of Lipofectamin 2000 (Invitrogen Company), at 37°C, 5% CO 2 Cells and medium were collected after culturing for 24 hrs. The culture medium was centrifuged and concentrated 50 times by ultrafiltration tube (Millipore Company). The signal of AMP-18 protein was detected by immunoblotting.
[0039] The result is as figure 2 As shown in A, the AMP-18 signal can be detected in both the EGFP-AMP-18 transfected cells and the medium, while the AMP-18 signal cannot be detected in the EV transfected cells and the medium. This shows that AMP-18 protein can be secreted by cells.
[0040] 2. Localization of exogenous AMP-18 by immunofluorescence experiment
[004...
Embodiment 3
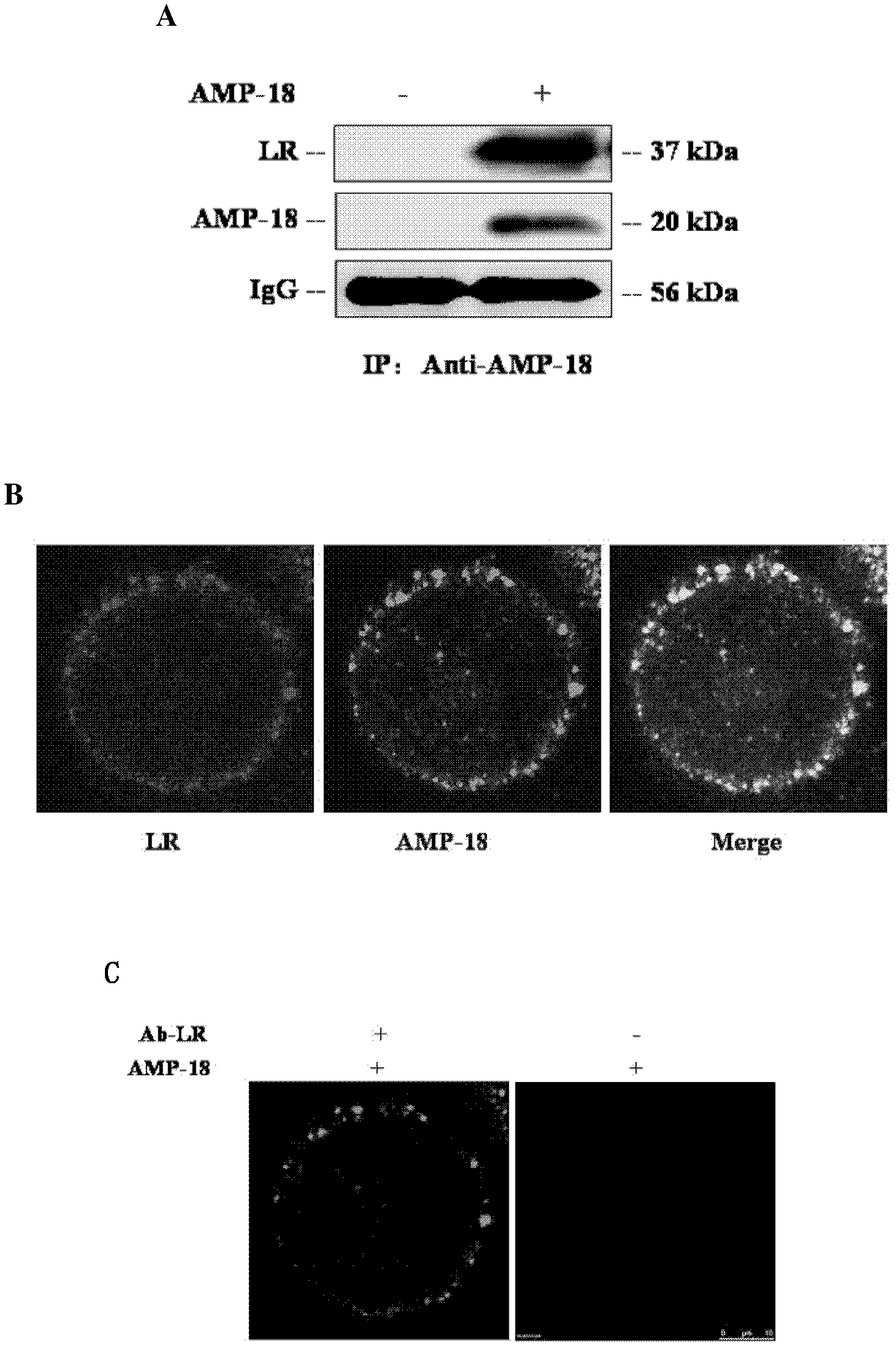
[0043] Example 3 LR is the receptor of AMP-18
[0044] 1. Co-immunoprecipitation method to verify the interaction between AMP-18 and LR
[0045] BGC-823 cells were treated with the recombinant AMP-18 prepared in Example 1 or the control solution for 5 minutes, and then the cell membrane proteins were extracted. Harvest about 5 x 10 7 Cells were washed twice with PBS and then incubated with 0.1XPBS at 4°C for 5min. Cells were collected and frozen and thawed five times in liquid nitrogen and 37°C water bath, 5 min each time. Centrifuge the cells at 35,000g at 4°C for 30min, take the supernatant and centrifuge at 150,000g at 4°C for 2hrs, and the pellet is the cell membrane fraction. and 1% protease inhibitor cocktail), lyse in ice bath for 30 min, centrifuge at 12,000 g at 4°C for 30 min, and transfer the supernatant to a new eppendorf tube. After quantification, take 3 mg of protein and dilute to 1 ml with lysate, add 40 μl of Protein A Sepharose column material that has be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com