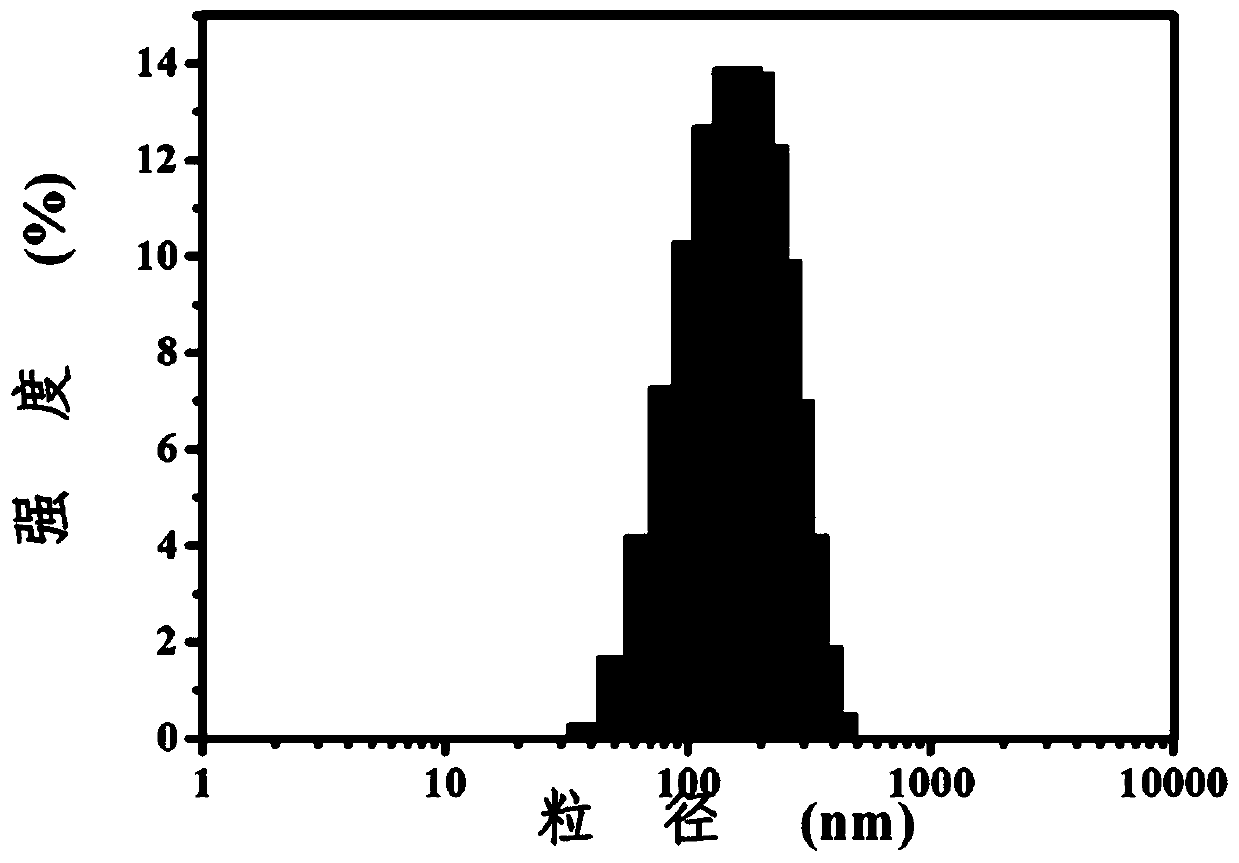
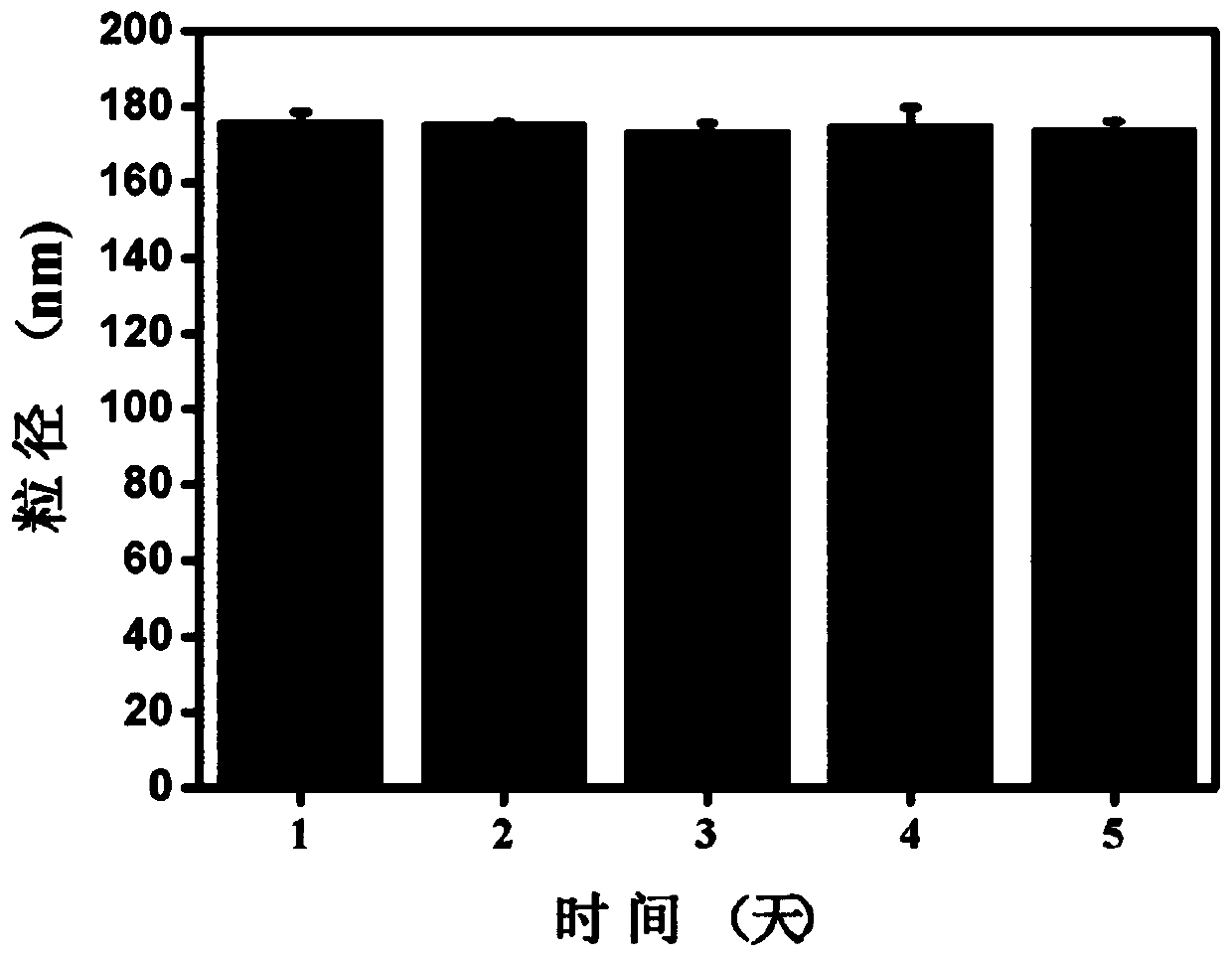
Nano-medicine, preparation method thereof and application of nano-medicine
A technology of nano-drugs and chemotherapeutic drugs, applied in the fields of nano-drugs, nano-technology, nano-technology, etc., can solve the problems of cytotoxicity, poor biocompatibility of gold nanorods, inability to carry drugs and photothermal agents, etc. Significant tumor and curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 nano drug
[0063] (1) Dissolve 0.1 mL of docetaxel with a concentration of 1 μg / mL in methanol, add 1 mL of sodium polyphosphate solution with a concentration of 20 mg / mL to obtain the first mixed solution; Calcium chloride solution was added to the first mixed solution to obtain the second mixed solution, which was centrifuged after 30 minutes of magnetic stirring at room temperature, and the precipitate was ultrasonically reconstituted to obtain the first dispersion, which contained docetaxel-encapsulated Calcium phosphate matrix;
[0064] (2) Add 10 mL of dopamine with a concentration of 1 mg / mL, adjust the pH to 8.5, centrifuge after 15 hours of magnetic stirring at room temperature, redissolve the precipitate and centrifuge, wash 3 times and redissolve the precipitate ultrasonically to obtain the second dispersion liquid. A calcium phosphate matrix containing surface-modified dopamine in the second dispersion;
[0065] (3) Add 1 m...
Embodiment 2
[0067] The preparation of embodiment 2 nano medicine
[0068] (1) Take 0.1 mL of paclitaxel with a concentration of 1.5 μg / mL and dissolve it in acetonitrile, add 1 mL of sodium polyphosphate solution with a concentration of 30 mg / mL to obtain the first mixed solution; The solution was added to the first mixed solution to obtain the second mixed solution, which was centrifuged after magnetic stirring at room temperature for 60 minutes, and the precipitate was ultrasonically redissolved to obtain the first dispersion, which contained paclitaxel (DTX) encapsulated calcium phosphate matrix;
[0069] (2) Add 10 mL of dopamine with a concentration of 2 mg / mL, adjust the pH to 9, centrifuge after 20 hours of magnetic stirring at room temperature, redissolve the precipitate and centrifuge, wash 4 times and redissolve the precipitate ultrasonically to obtain the second dispersion liquid. A calcium phosphate matrix containing surface-modified dopamine in the second dispersion;
[007...
Embodiment 3
[0072] The preparation of embodiment 3 nano medicine
[0073] (1) Dissolve 0.1 mL of camptothecin with a concentration of 0.5 μg / mL in dimethyl sulfoxide, and add 1 mL of sodium polyphosphate solution with a concentration of 10 mg / mL to obtain the first mixed solution; Calcium chloride solution per mL was added to the first mixed solution to obtain the second mixed solution, which was centrifuged after magnetic stirring at room temperature for 10 minutes, and the precipitate was ultrasonically redissolved to obtain the first dispersion, which contained Calcium phosphate matrix of resin;
[0074] (2) Add 10 mL of dopamine with a concentration of 0.5 mg / mL, adjust the pH to 7, centrifuge after magnetic stirring at room temperature for 10 h, redissolve the precipitate and centrifuge, wash twice, and redissolve the precipitate ultrasonically to obtain the second dispersion. A calcium phosphate matrix containing surface-modified dopamine in the second dispersion liquid;
[0075] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com