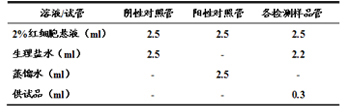
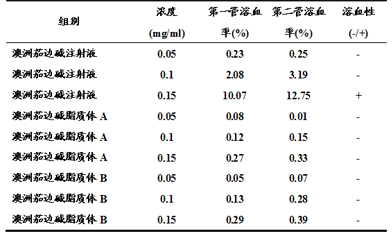
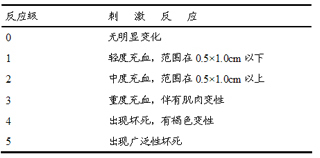
Solamargine liposome, preparation method and usage thereof
A technology of solanine liposomes and solanines, which is applied in the field of alkaloid liposomes, can solve problems such as hemolysis and other toxic side effects, application obstacles, etc., achieve improved anti-tumor effects, improve muscle and blood vessel stimulation, and facilitate industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Example 1. Solanine liposome preparation.
[0166] Party:
[0167] Australian solanine 0.1g
[0168] Soy Lecithin for Injection 1.5g
[0169] Cholesterol 0.4g
[0170] Tween-80 0.2g
[0171] pH=4.9 phosphate buffered saline solution 100ml
[0172] Weigh the soybean lecithin and cholesterol for injection of the prescribed amount and dissolve them in 300ml of ether, dissolve solanine in 100ml of phosphate buffered saline solution, mix the organic phase and the aqueous phase evenly, then add the prescribed amount of Tween-80, Heated in a water bath to 35°C, treated with ultrasound for 15 minutes, evaporated the organic solvent under reduced pressure until there was no ether smell, and then dispersed it with ultrasound to obtain solanine liposomes with an encapsulation efficiency of 61.5%.
Embodiment 2
[0173] Example 2. Solanine liposome preparation.
[0174] Party:
[0175] Australian solanine 0.1g
[0176] Soy Lecithin for Injection 1.5g
[0177] Cholesterol 0.4g
[0178] Tween-80 0.2g
[0179] pH=4.9 phosphate buffered saline solution 100ml
[0180] Weigh the prescribed amount of soybean lecithin and cholesterol for injection, dissolve in 300ml chloroform, transfer to a round bottom flask, and at a water bath temperature of 40°C, the rotation speed is 150r / min, and the organic solvent is evaporated under reduced pressure to form a film. After formation, the organic solvent was completely removed by nitrogen gas for several minutes. Weigh the prescription amount of solanine and Tween-80 and dissolve it in 100ml of phosphate buffered saline solution, transfer the buffer solution into a flask, spin and wash the membrane at room temperature and normal pressure until the membrane completely falls off, hydrate for 30min, and then sonicate After 20 minutes of treatment, s...
Embodiment 3
[0181] Example 3. Solanine liposome preparation.
[0182] Party:
[0183] Australian solanine 0.1g
[0184] Soy Lecithin for Injection 1.5g
[0185] Cholesterol 0.4g
[0186] Poloxamer F-68 0.2g
[0187] pH=4.9 phosphate buffered saline solution 100ml
[0188] Weigh the prescribed amount of soybean lecithin for injection and cholesterol, dissolve in 300ml ether, dissolve poloxamer F-68, and Australian solanine in 100ml phosphate buffered saline solution, mix the organic phase and the aqueous phase evenly, and heat in a water bath to 35°C, sonicate for 15 minutes, evaporate the organic solvent under reduced pressure until there is no ether smell, and then disperse it by sonication to obtain solanine liposomes with an encapsulation efficiency of 70.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com