Synthesis process of thiazole substituted pyrimidine compound
A synthesis process and compound technology, which is applied in the field of synthesis of polybasic substituted pyrimidines in pharmaceutical intermediates, can solve the problems of low yield of target products and multiple by-products, and achieve improved selectivity and controllability of operation, high yield synthesis , produce controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
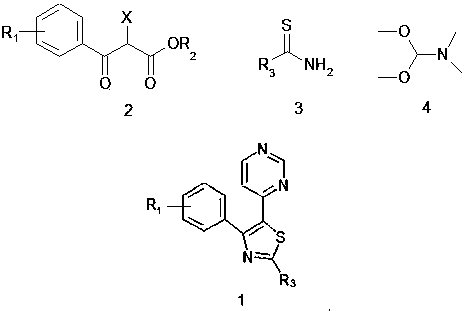
[0039] A synthesis process for thiazole-substituted pyrimidine compounds represented by the following formula (1), comprising the following steps:
[0040] Thiazole ring closure reaction
[0041] The compound represented by the following formula (2) (6g, 1.2mol) and the compound represented by the following formula (3) (6.5g, 1.7mol) in the accelerator bistrifluoromethanesulfonylimide silver salt (0.9g, 0.6mol) , catalyst β-cyclodextrin (1g, 1.2mol), co-catalyst phenylphosphine gold chloride (0.15g, 0.24mol), in N,N-dimethylformamide (DMF) (40ml) and In 1,4-dioxane (13.5ml) mixed solvent, magnetically stirred for 4 hours, thiazole ring closure reaction occurred, and the reaction was completed by TLC detection;
[0042] pyrimidine cyclization reaction
[0043] The reaction system after the thiazole ring closure reaction was first acetylated, and then the compound N,N-dimethylformamide dimethyl acetal (DMF-DMA) (7.5g, 1.2mol), heated to 105°C under the action of additive guan...
Embodiment 2
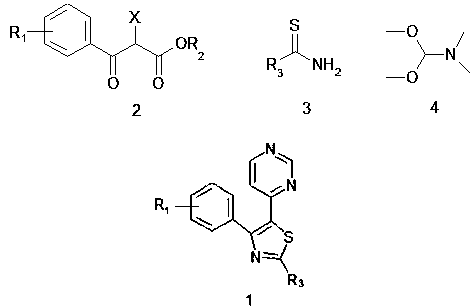
[0051] A synthesis process for thiazole-substituted pyrimidine compounds represented by the following formula (1), comprising the following steps:
[0052] Thiazole ring closure reaction
[0053]The compound represented by the following formula (2) (6g, 1.2mol) and the compound represented by the following formula (3) (6.9g, 1.8mol) in the accelerator bistrifluoromethanesulfonimide silver salt (1.05g, 0.96mol) , catalyst β-cyclodextrin (1.08g, 1.4mol), co-catalyst phenylphosphine gold chloride (0.22g, 0.36mol), in N,N-dimethylformamide (DMF) (45ml) In a mixed solvent with 1,4-dioxane (15ml), magnetically stir the reaction for 4 hours, a thiazole ring closure reaction occurs, and the reaction is completed by TLC detection;
[0054] pyrimidine cyclization reaction
[0055] The reaction system after the thiazole ring closure reaction is first acetylated, and then the compound N,N-dimethylformamide dimethyl acetal (DMF-DMA) (8g, 1.43 mol), heated to 120°C under the action of ad...
Embodiment 3
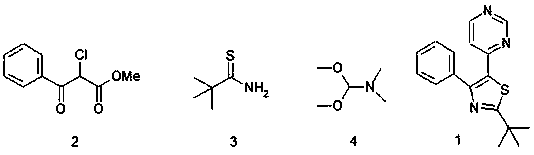
[0063] A synthesis process for thiazole-substituted pyrimidine compounds represented by the following formula (1), comprising the following steps:
[0064] Thiazole ring closure reaction
[0065] The compound represented by the following formula (2) (6g, 1.2mol) and the compound represented by the following formula (3) (7.1g, 2.1mol) in the accelerator bistrifluoromethanesulfonimide silver salt (1g, 0.84mol), Under the action of catalyst β-cyclodextrin (1.2g, 1.5mol) and cocatalyst phenylphosphine gold chloride (0.26g, 0.43mol), in N,N-dimethylformamide (DMF) (45ml) and In 1,4-dioxane (15ml) mixed solvent, magnetically stirred for 4 hours, thiazole ring closure reaction occurred, and TLC detected that the reaction was complete;
[0066] pyrimidine cyclization reaction
[0067] The reaction system after the thiazole ring closure reaction was first acetylated, and then the compound N,N-dimethylformamide dimethyl acetal (DMF-DMA) (11.2g, 2.16mol), heated to 140°C under the act...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com