Liposome including taxane compound
A compound, taxane technology, applied in the field of liposomes encapsulating taxane compounds, can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
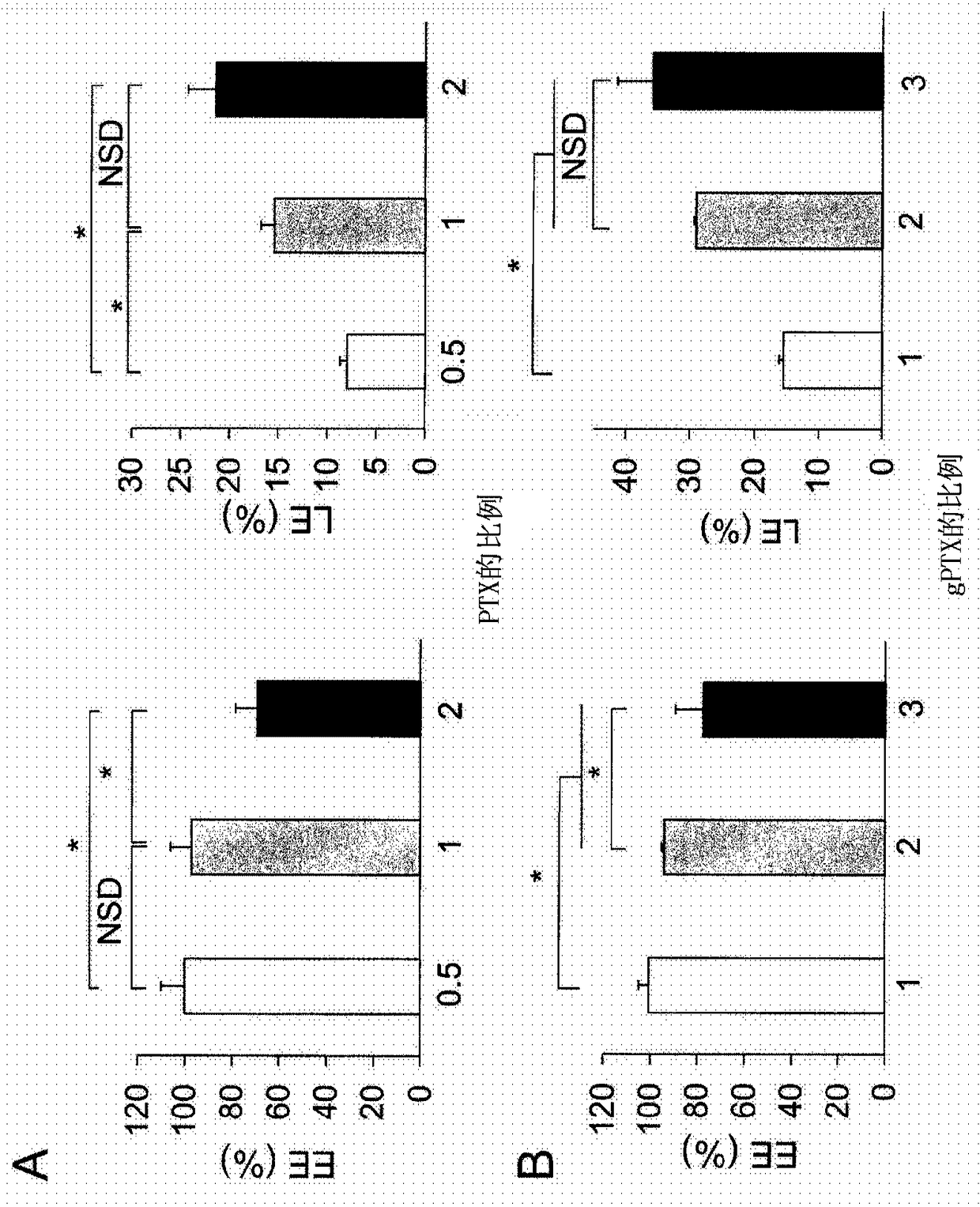
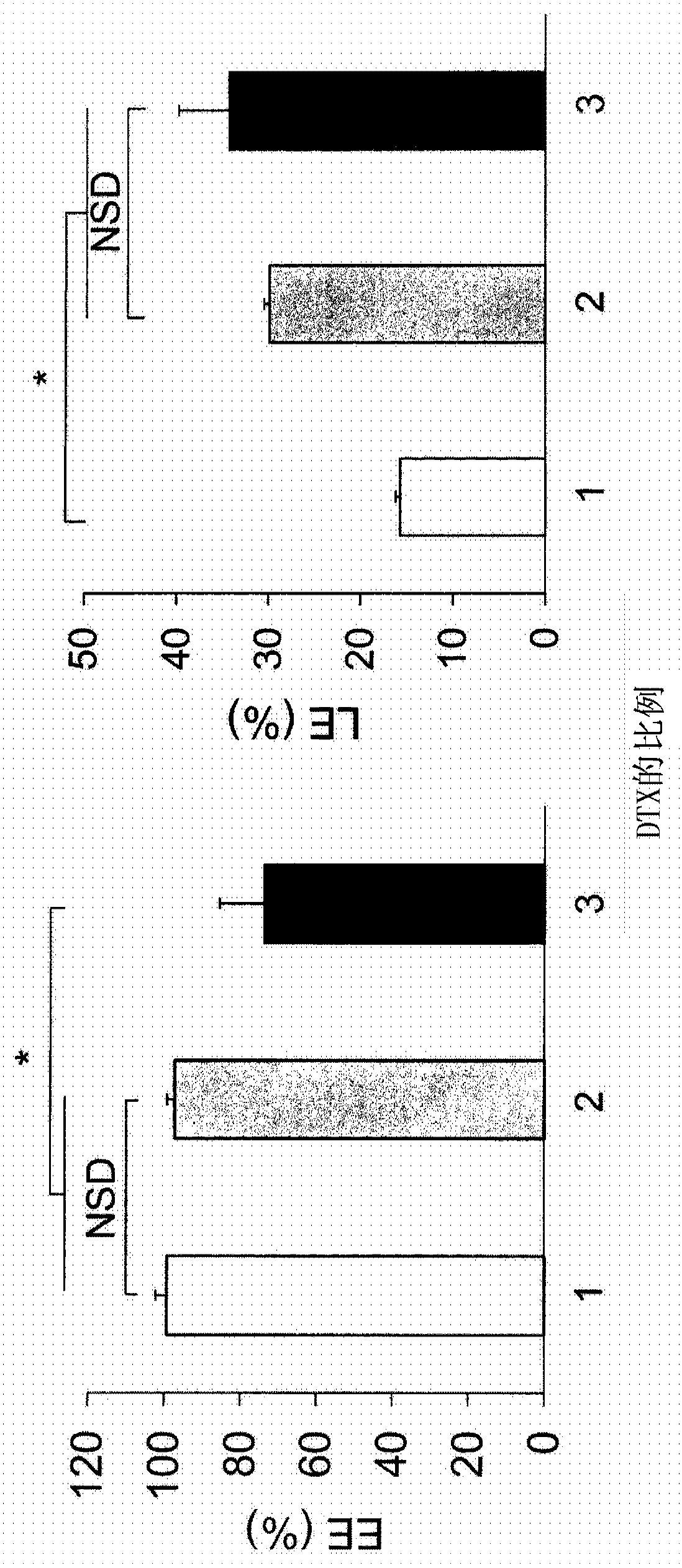
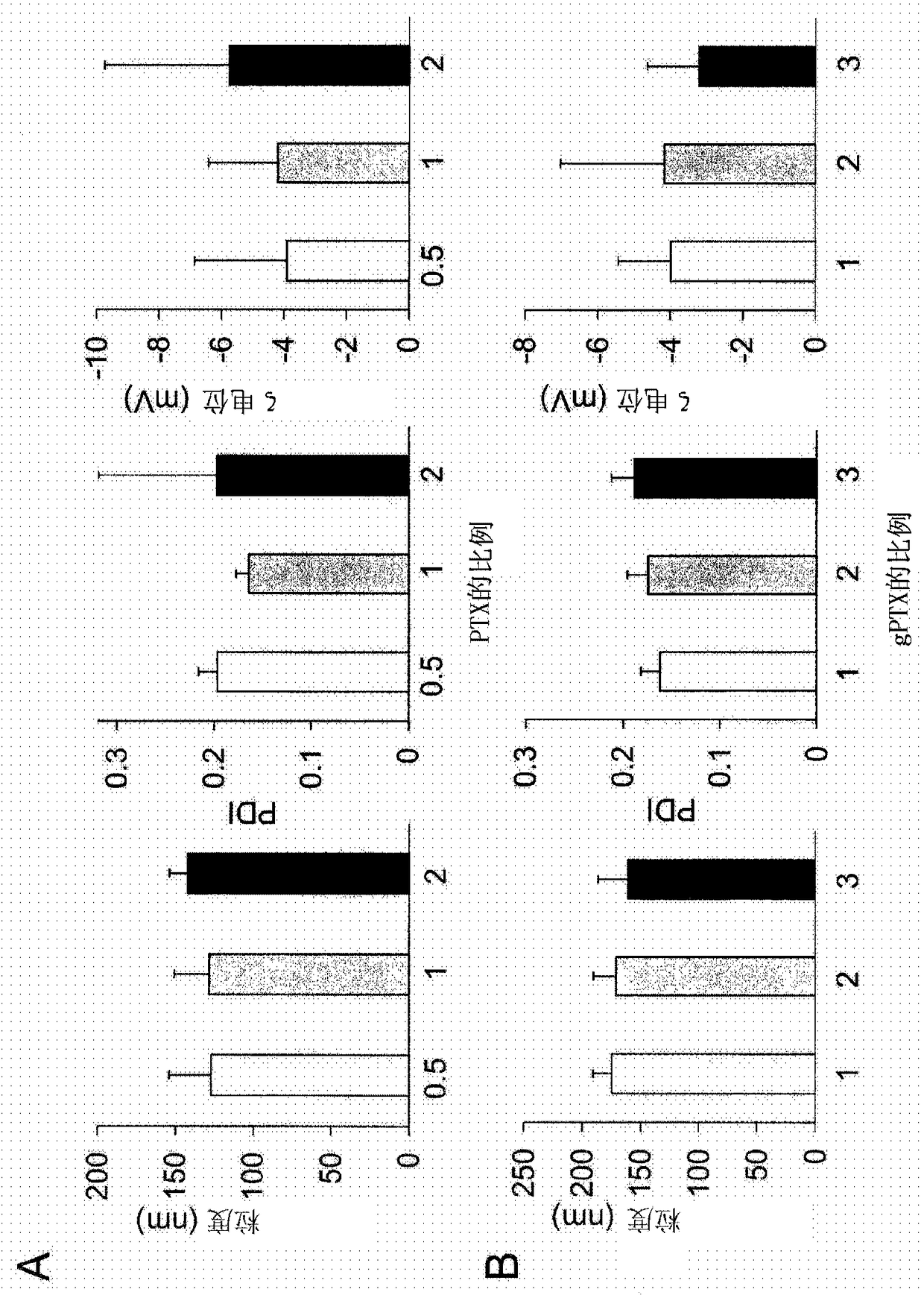
[0207] Liposomes Encapsulating PTX, gPTX, or DTX
[0208] Liposomes encapsulating PTX (paclitaxel), gPTX (7-glucosyloxyacetylpaclitaxel) or DTX (docetaxel) were prepared by thin film hydration method.
[0209] Weigh 9.6 mg hydrogenated soybean lecithin (HPSC), 3.2 mg cholesterol (Chol), 3.2 mg 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxyl (polyethylene glycol) -2000] (mPEG-DSPE) and taxane compound (PTX, gPTX or DTX) and add to the eggplant-shaped flask with the molar ratio of HSPC:Chol:mPEG-DSPE:taxane compound=6:4:0.5:X .
[0210] Specifically, weigh 0.9 mg, 1.8 mg or 3.5 mg (x=0.5 to 2) of PTX, 2.2 mg, 4.4 mg or 6.6 mg (x=1 to 3) of gPTX and 1.7 mg, 3.3 mg or 5.0 mg (x= 1 to 3) DTX and add to eggplant flask.
[0211] 4 mL of an organic solvent (chloroform:methanol=9:1) was added to the eggplant-shaped flask, and the mixed lipids were sufficiently dissolved. Then, the mixed lipids were vacuum-dried using a rotary evaporator to completely remove the organi...
preparation Embodiment 2
[0219] Liposomes Encapsulating Bafilomycin A1 (BafA1)
[0220]Liposomes encapsulating BafA1 were prepared by thin film hydration method. 9.6 mg HSPC, 3.2 mg Chol, 3.2 mg mPEG-DSPE and BafA1 were weighed and added to an eggplant-shaped flask at a molar ratio of HSPC:Chol:mPEG-DSPE=6:4:0.5:x.
[0221] 145 μg or 290 μg (x=0.1 or 0.2) of BafAl was weighed and added thereto.
[0222] 4 mL of an organic solvent (chloroform:methanol=9:1) was added to the eggplant-shaped flask, and the mixed lipids were sufficiently dissolved. Then, the mixed lipids were vacuum-dried using a rotary evaporator to completely remove the organic solvent and form a lipid film encapsulating BafA1.
[0223] The liposome formation process using the thus-produced BafA1-encapsulating lipid film and the evaluation of the properties of the liposome were performed in the same manner as in the preparation of the taxane compound-encapsulating liposome.
[0224] The concentration of BafA1 encapsulated in liposom...
Embodiment 2
[0231] Cytotoxicity Assessment
[0232] Cytotoxicity of liposomes encapsulating PTX, gPTX or DTX was evaluated by using the MTT assay. As test target cells, the cell line HT-29 cells derived from human colon cancer, the cell line SK-OV-3 cells derived from human ovarian cancer, and the cell line SK-BR-3 cells derived from human breast cancer were used. Cancer cells were seeded into 96-well plates at 5000 cells / well.
[0233] After 24 hours of incubation, different concentrations of drugs were added to each well. After 72 hours of drug exposure, MTT solution was added at a final concentration of 0.5 mg / mL, followed by incubation for 4 hours. Then, the resulting A dissolved in nail Solution in liquid (10% SDS + 0.02-adjusted HCl). Calculate the concentration that causes 50% cell death (IC 50 ).
[0234] The cytotoxicity of BafA1 and liposomes encapsulating BafA1 was evaluated by using the following MTT assay. As test target cells, the cell line HT-29 cells derived fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com