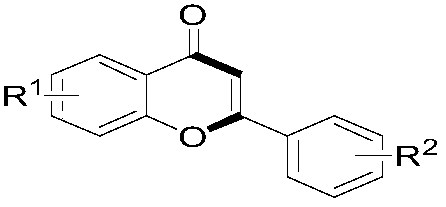
Preparation method of 2-aryl flavone
A technology of aryl flavonoids and hydroxy aryl formic acid, which is applied in the field of preparation of 2-aryl flavonoids, can solve the problems of rare alkynylation reaction and the like, and achieves cheap and easy-to-obtain catalysts, mild reaction conditions and cheap reagents easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
[0030] O-hydroxyarylformylformic acid (1.0mmol, R 1 =H), aryl alkynoic acid (1.1mmol, R 2 =H), silver catalyst (5mol%) and oxidizing agent (2mmol) were dispersed in solvent (2mL), then stirred at 80°C for 3 hours under air atmosphere. After the reaction, with Et 2 O (5 mL) was extracted three times, and the combined organic phases were concentrated under reduced pressure. The obtained crude product was subjected to column chromatography (300-400 mesh silica gel, petroleum ether and ethyl acetate as eluents) to obtain the target product. The types of silver catalysts, oxidants and solvents used and the reaction results are shown in Table 1.
[0031] The reaction condition and reaction result of table 1 embodiment 1~9
[0032]
[0033] a Solvent weight kept at 2mL
Embodiment 10~29
[0035] O-hydroxyarylformylformic acid (1.0mmol), aryl alkynoic acid (1.1mmol), AgNO 3 (5mol%) and K 2 S 2 o 8 (2 mmol) was dispersed in a mixed solvent of acetonitrile (1.5 mL) and water (0.5 mL), and stirred at 80° C. for 3 hours under an air atmosphere. After the reaction, with Et 2 O (5 mL) was extracted three times, and the combined organic phases were concentrated under reduced pressure. The obtained crude product was subjected to column chromatography (300-400 mesh silica gel, petroleum ether and ethyl acetate as eluents) to obtain the target product. The o-hydroxyarylformylformic acid and aryl alkynoic acid used and the reaction results are shown in Table 2.
[0036] The reaction conditions and reaction result of table 2 embodiment 10~29
[0037]
[0038] The characterization data of some products are as follows:
[0039] 2-Phenyl-4H-chromen-4-one(5a): Yellow solid. 1 H NMR (400MHz, CDCl 3 )δ8.25(d,J=7.6Hz,1H),7.97–7.91(m,2H),7.70(t,J=7.6Hz,1H),7.56(dd,J=17.2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com