A method for preparing neoastilbin with Smilax cocos as raw material
A technology for new astilbin and tuckahoe, which is applied in the field of preparation of new astilbin and can solve the problems of lack and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
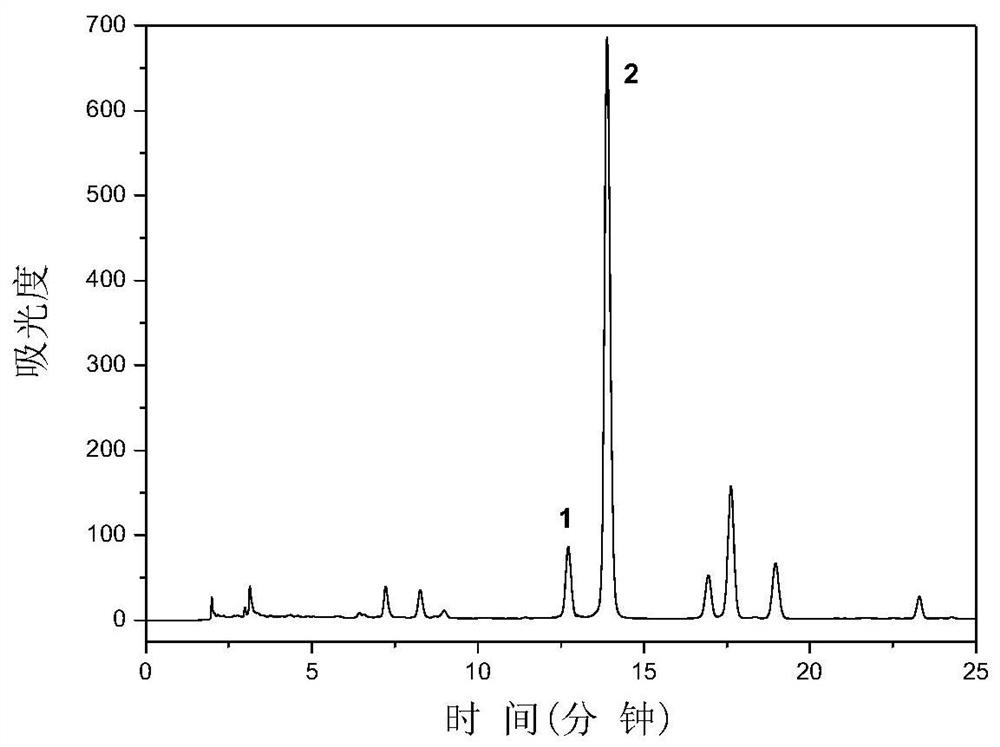
[0031] 1 kg of Smilax smilax was crushed and passed through a 40-mesh sieve, 20 L of 50% ethanol aqueous solution was added, stirred and extracted at room temperature for 60 minutes, and the extract was obtained by conventional filtration. figure 1 High performance liquid chromatography for Smilax smilax extract. In the figure, peak 1 is astilbin, and peak 2 is astilbin. As determined by high performance liquid chromatography, the content of astilbin in Smilax cocos is 2.2%, and the content of neoastilbin is 0.17%, that is, 1kg of Smilax cocos contains 22g of astilbin and 1.7g of neoastilbin; The content of astilbin was the highest, while the content of astilbin was very low.
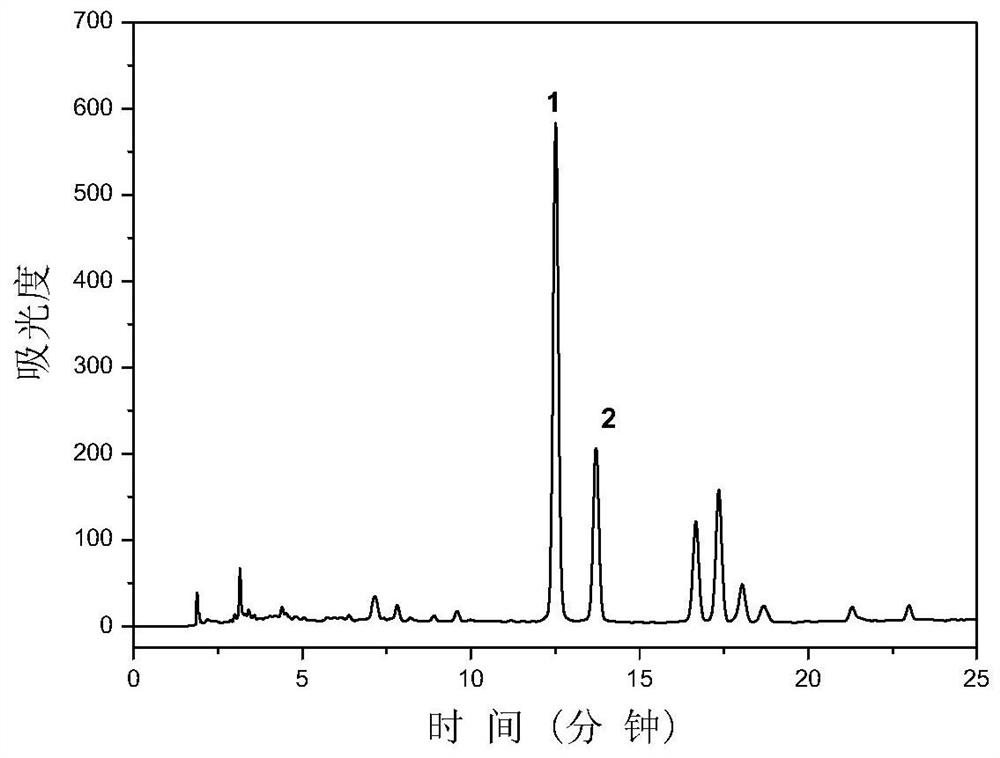
[0032] Concentrate the Smilax smilax extract to remove ethanol, adjust the pH to 8, add ascorbic acid with a final concentration of 1 mM, and place it in an 80-degree water bath for 10 hours to carry out isomerization reaction. figure 2 It is high performance liquid chromatography of Smilax smilax ex...
Embodiment 2
[0037] (1) Take 1 kg of Smilax cocos and crush it through a 40-mesh sieve, add 20 L of 60% ethanol solution, stir and extract at room temperature for 60 minutes, and filter through a 200-mesh filter cloth to obtain the extract.
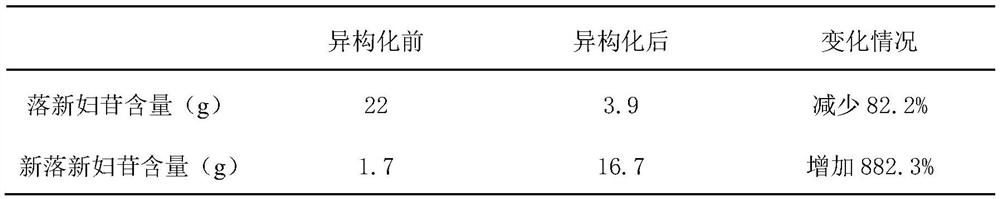
[0038] (2) The temperature of the extract obtained in step (1) is concentrated under vacuum at 60 degrees, and the ethanol is recovered to obtain 4L of the concentrated solution; as determined by high performance liquid chromatography, the concentrated solution contains 22 g of astilbin and 1.7 g of astilbin in total. g.
[0039] (3) After adjusting the pH of the concentrated solution obtained in step (2) to 7 with 1mol / L NaOH solution, add 0.704g of ascorbic acid, the final concentration of ascorbic acid is 1mM, and place it in an 80°C water bath for 10 hours to carry out isomerization reaction. As determined by high performance liquid chromatography, the concentrated solution now contained 3.9 g of astilbin and 16.7 g of astilbin in total. The conc...
Embodiment 3
[0043] A method for preparing neoastilbin with Smilax cocos as raw material, comprising the following steps:
[0044] 1) get Smilax smilax, cross 20 mesh sieves after pulverizing, take the ethanol aqueous solution that concentration is 10% (v / v) as extractant, be 1:20 ( kg:L), the sieve was mixed with the extractant, stirred at room temperature for 60min, filtered, and the filtrate was taken;
[0045] 2) concentrating the filtrate obtained in step 1) in a vacuum at 60° C., recovering ethanol, and obtaining a concentrated solution;
[0046] 3) The pH of the concentrated solution obtained in step 2) was adjusted to 5, and ascorbic acid was added thereto to a final concentration of 0.1 mM, and left to react at 50° C. for 16 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com