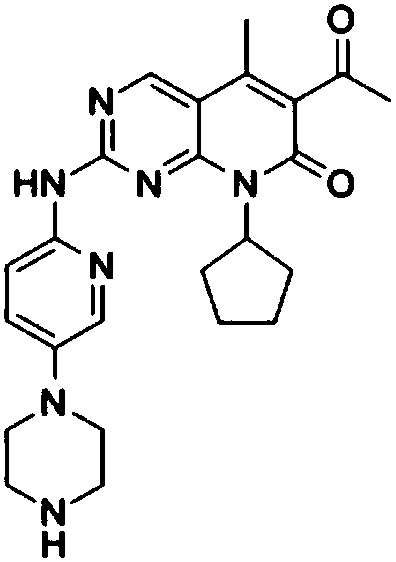
Palbociclib oral emulsion and preparation method thereof
A technology of emulsion and emulsifier, which is applied in the field of oral emulsion of palbociclib and its preparation, can solve the problems of high oral absorption and impact of palbociclib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
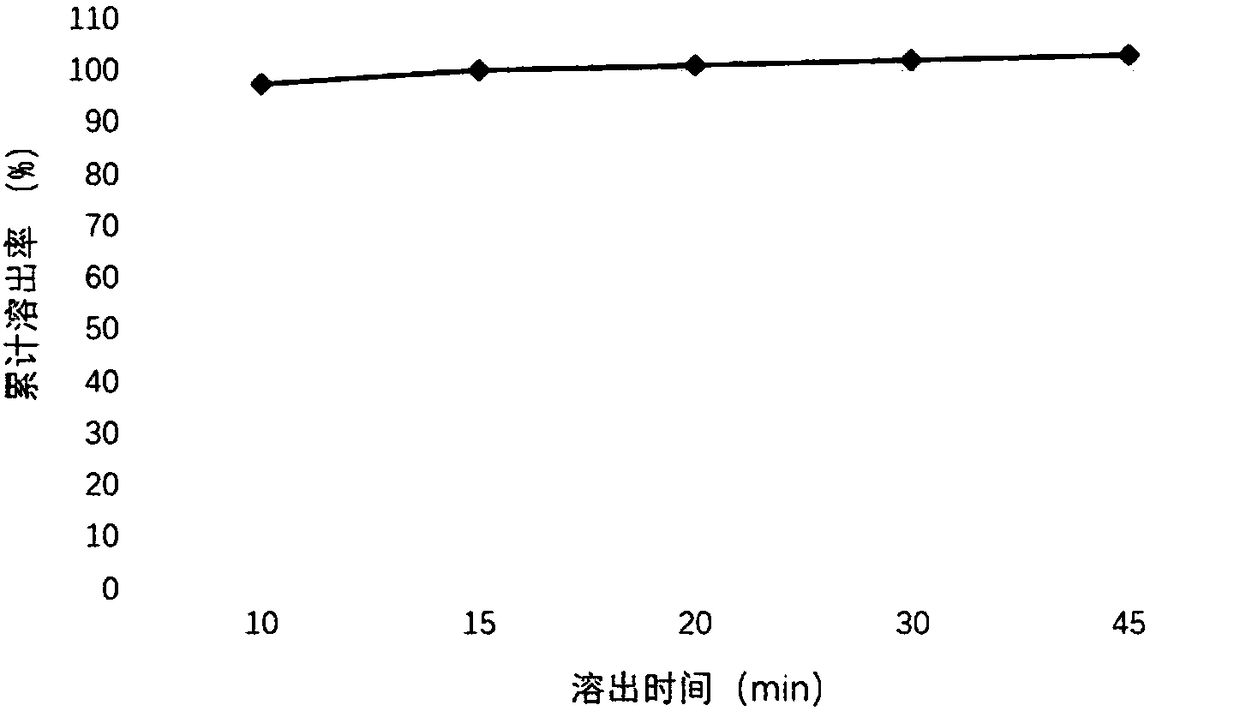
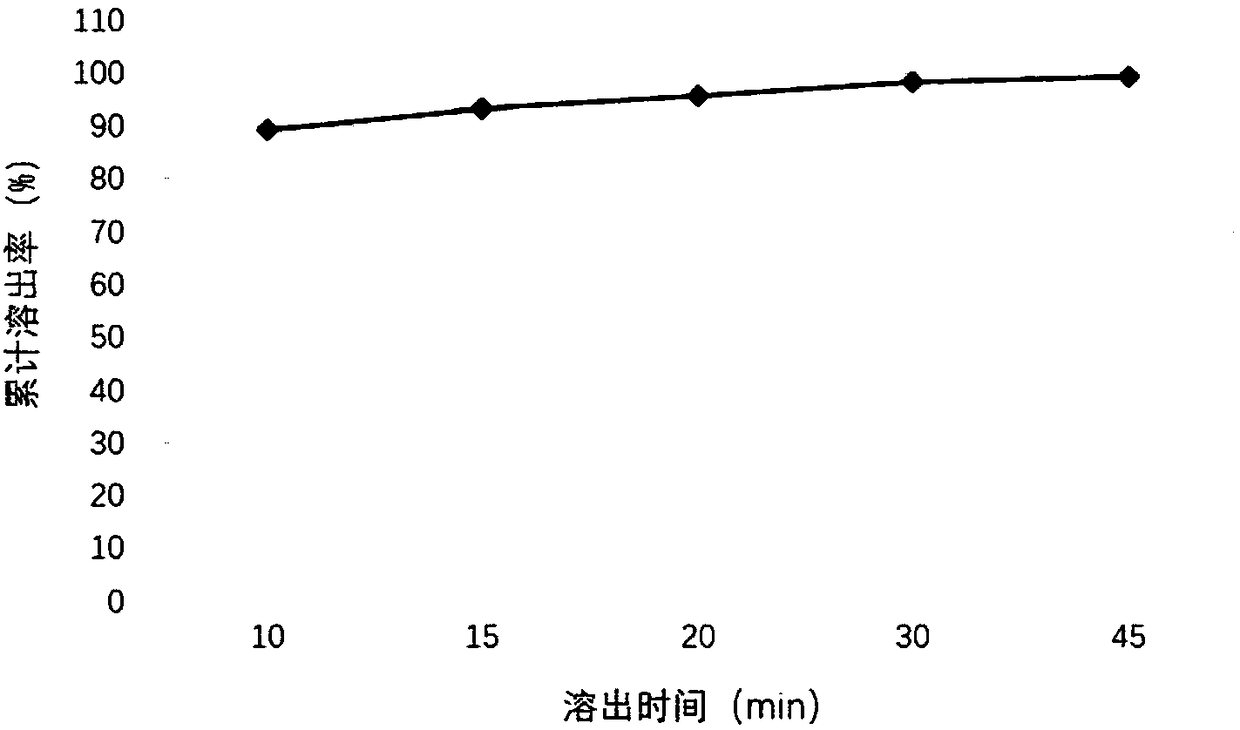
[0040] (1) Weigh 150 mg of tartaric acid and dissolve it in 20 mL of purified water, stir and dissolve completely to obtain a clear and transparent solution; (2) Weigh 125 mg of palbociclib free base and add it to the tartaric acid aqueous solution prepared in the previous step, and stir until completely dissolved; (3) Add 100 mg of sucralose, 5 mL of caprylic capric macrogolglyceride, 4.75 mL of polyethylene glycol 400 and 20 mg of sodium benzoate to the above solution, and stir to dissolve it completely; (4) Add 0.6g of corn oil, 20mg of butylated hydroxytoluene and 20mg of butylated hydroxyanisole were stirred by a high-speed stirrer at a speed of 1000rpm for 10min to obtain the product.
Embodiment 2
[0042] (1) Weigh 100mg of citric acid and 100mg of tartaric acid and dissolve them in 20mL of purified water, stir and dissolve completely to obtain a clear and transparent solution; (2) Weigh 125mg of palbociclib free base and add citric acid and tartaric acid prepared in the previous step Mix the aqueous solution and stir until it dissolves completely; (3) Add 100 mg of aspartame, 9 mL of caprylic capric macrogol glyceride, 2 mL of polyethylene glycol 400 and 20 mg of sodium benzoate to the above solution, and stir to make it completely dissolve; (4) Add 1.5 g of coconut oil and 40 mg of vitamin E to the above aqueous solution, and stir for 10 min at a speed of 1000 rpm with a high-speed stirrer to obtain the product.
Embodiment 3
[0044] (1) Weigh 100 mg of malic acid and dissolve it in 20 mL of purified water, stir and dissolve completely to obtain a clear and transparent solution; (2) Weigh 75 mg of palbociclib free base and add it into the malic acid aqueous solution prepared in the previous step, and stir until dissolved Completely; (3) Add 150 mg of sodium saccharin, 5 mL of polyoxyethylene castor oil, 4.75 mL of polyethylene glycol 400 and 10 mg of sodium benzoate to the above solution, and stir to dissolve it completely; (4) Add 0.1 g of oil to the above aqueous solution Acid and 40 mg of vitamin E were stirred for 10 minutes at a speed of 1000 rpm by a high-speed stirrer to obtain the product.
[0045] Table 1 and Table 2 below show the solubility of palbociclib in aqueous solutions of different pH and in different oils, respectively.
[0046] Table 1. Solubility of palbociclib free base in different buffer solvents
[0047]
[0048] Table 2. Solubility of 20mg palbociclib free base in diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com