MiRNA-supported composite nanoparticle as well as preparation method and application thereof
A technology of composite nanoparticles and membrane materials, applied in the field of biomedicine, can solve the problems of non-degradation and metabolism, poor biocompatibility, complex preparation, etc., to promote selective release and gene regulation, and improve targeting accuracy Sexuality and efficiency, enhance the effect of gene regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
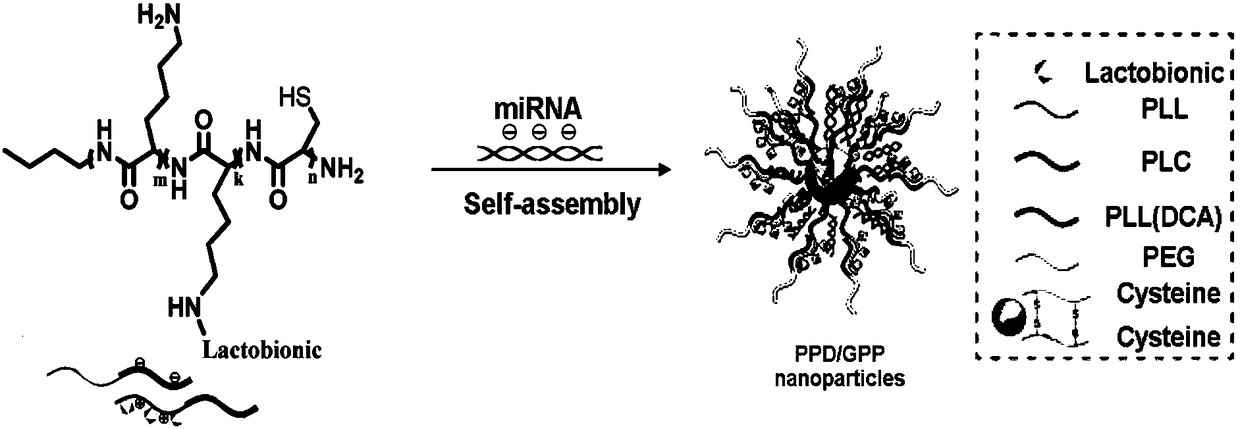
[0061] The preparation of embodiment 1 galactose-polylysine-polycysteine polymer Gal-PLL-PLC (GPP)
[0062] Dissolve n-butane (12.58 μL) and Lys(Z)-NCA (0.78 g) into 20 mL of dimethylformamide DMF, stir at 30° C. under nitrogen protection, and react for 72 hours. After the reaction, add 5 to 50 times of diethyl ether to precipitate, filter and dry to obtain PLL(Z).
[0063] Through the method of NCA ring-opening polymerization, the initiator PLL(Z) (0.41g) and Cys(Z)-NCA (0.22g) were heated to 30°C under the protection of nitrogen, and reacted at constant temperature for 48 hours to synthesize polylysine Acid-polycysteine (PLL(Z)-PLC(Z)) diblock polymer. Dissolve the PLL(Z)-PLC(Z) diblock polymer in trifluoroacetic acid at 0°C, add 30% HBr / HAc to react for 2 hours, add 10 times of diethyl ether to precipitate, filter, and dissolve the obtained product in Polar organic solvents were dialyzed in water for 48 hours using a dialysis bag with a molecular weight cut-off of 350...
Embodiment 2
[0065] Embodiment 2 has the preparation of the polyethylene glycol-polylysine polymer of acid-sensitive effect
[0066] Ring-opening polymerization via NCA with initiator PEG-NH 2 (0.4g) reacted with Lys(Z)-NCA (0.49g) at constant temperature for 48 hours under the protection of nitrogen to synthesize PEG-PLL polymer. 3,4,5,6-tetrahydrophthalic anhydride DCA (49 mg) was added to the PEG-PLL solution (5.7 mg, 10 mg / mL) and stirred in the dark at 25°C for 2 hours. Add 3M NaOH to the above mixture to maintain pH8.5, dialyze for 48 hours, change the dialysis water every 2 hours, and then freeze-dry to obtain PEG-PLL (DCA), that is, PPD.
Embodiment 3
[0067] Example 3 Preparation of composite nanoparticles loaded with microRNA:
[0068] Combine the synthesized microRNA-CY3 with galactose-polylysine-polycysteine polymer and the polyethylene glycol-polylysine polymer aqueous solution (microRNA:GPP:PPD=1:40: 40) mixed and oscillated, and left to stand at room temperature for 5 minutes to obtain miRNA-loaded composite nanoparticles ( figure 1 ). The particle size distribution and electron microscope characterization are as follows Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com