Synthetic method of fatty acid containing nitrogen heterocycle
A synthesis method and fatty acid technology, applied in chemical instruments and methods, carboxylate preparation, organic compound preparation, etc., can solve problems such as difficult storage, no practical application value, high price of organometallic reagents, etc. The method is simple and reliable Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
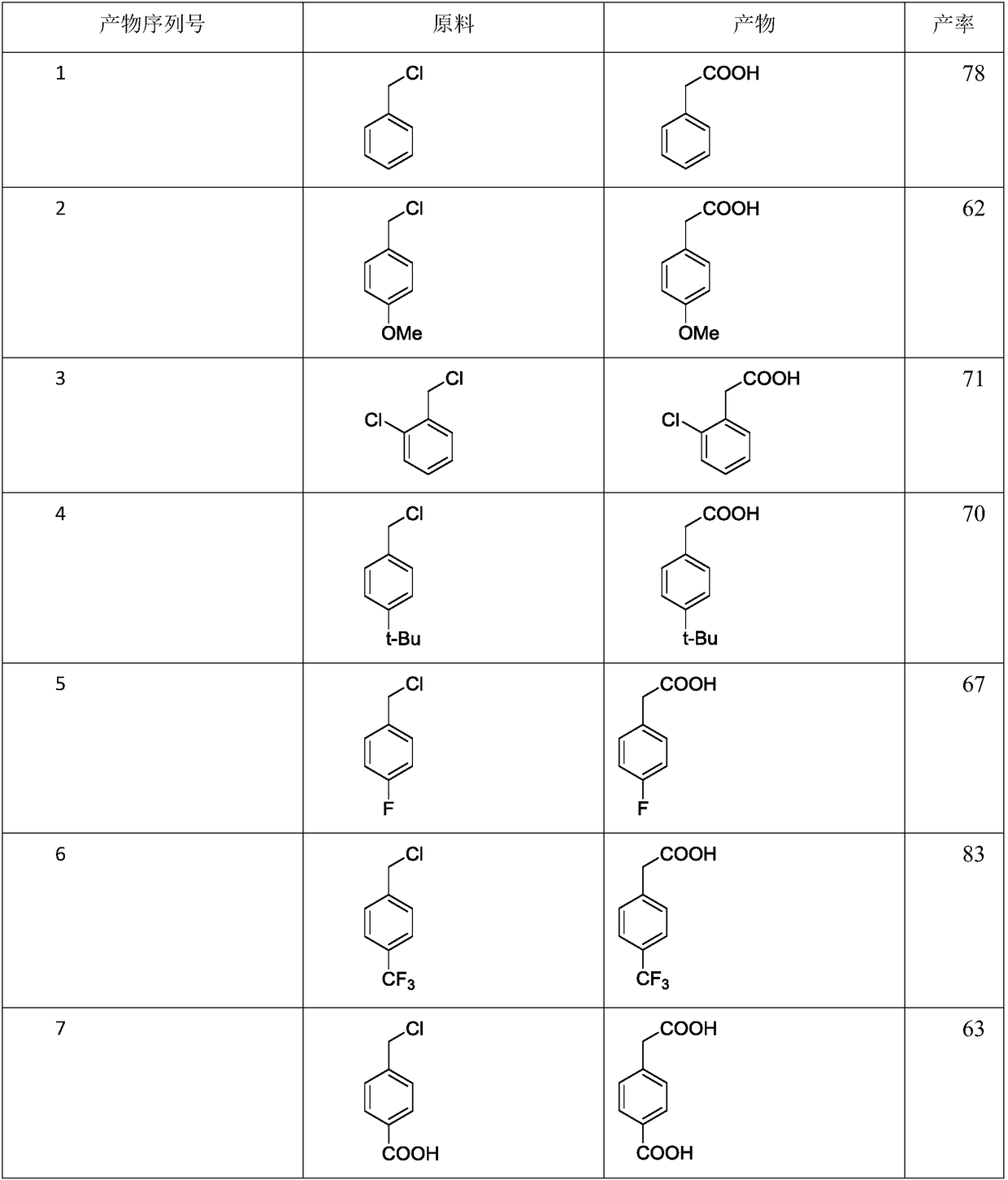
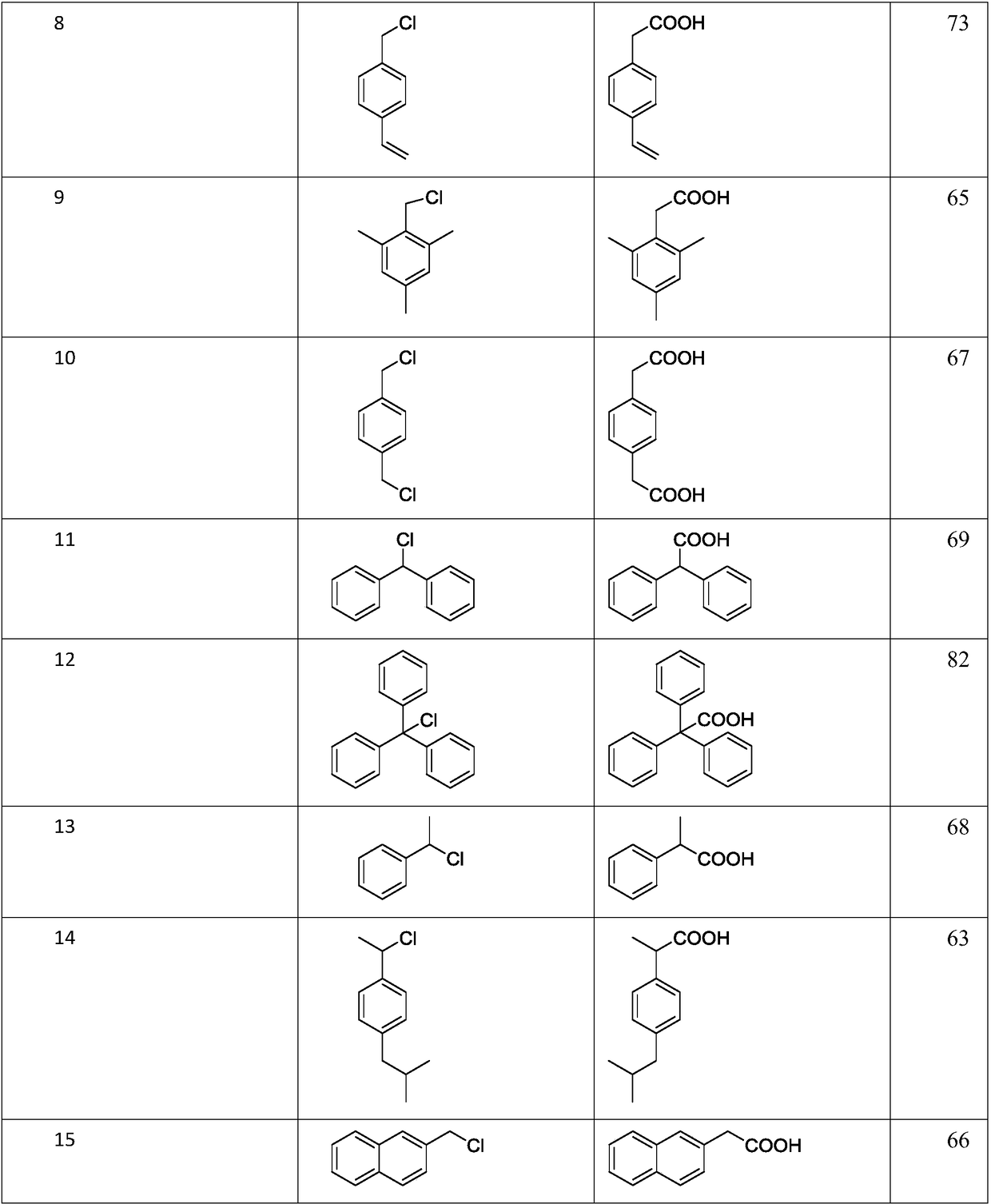
Image
Examples
Embodiment 1
[0016] The synthetic method of 6-chloro-3-pyridine acetic acid, comprises the following steps:
[0017] S1: take 8.72mmol 2-chloro-5-chloromethylpyridine Catalyst and solvent DMF are added to the reactor;
[0018] S2: Introduce carbon dioxide to make the pressure in the kettle 3 MPa, adjust the reaction at 40° C. for 15 hours.
[0019] S3: add dilute hydrochloric acid in the reaction kettle to acidify, extract with ethyl acetate, combine the organic phases, remove the liquid by rotary evaporation, and further vacuum dry to obtain 6-chloro-3-pyridineacetic acid
[0020] The catalyst includes zinc powder and lithium chloride, chloromethyl heterocyclic compound and zinc powder, and the molar ratio of lithium chloride to the reactor is 1:3:3, and every mmol of 2-chloro-5-chloromethylpyridine Add 2.8-2.9ml solvent DMF.
[0021] The nuclear magnetic resonance (1H NMR and 13C NMR) detection data of the compound 6-chloro-3-pyridineacetic acid of embodiment 1 is:
[0022] 1H NMR...
Embodiment 2
[0029] The synthetic method of 2-chloro-5-thiazole acetic acid, comprises the following steps:
[0030] S1: Will take 8.72mmol 2-chloro-5-chloromethylthiazole Catalyst and solvent DMF are added to the reactor.
[0031] S2: Introduce carbon dioxide to make the pressure in the kettle 4MPa, and adjust the reaction at 40°C for 16 hours;
[0032] S3: add dilute hydrochloric acid in the reaction kettle to acidify, extract with ethyl acetate, combine the organic phases, remove the liquid by rotary evaporation, and further vacuum dry to obtain 2-chloro-5-thiazoleacetic acid
[0033] The catalyst includes zinc powder and lithium chloride, chloromethyl heterocyclic compound and zinc powder, and the molar ratio of lithium chloride to the reactor is 1:3:3, and every mmol of 2-chloro-5-chloromethylthiazole 2.8 ml solvent DMF was added.
[0034] The nuclear magnetic resonance (NMR) of the compound 2-chloro-5-thiazole acetic acid of embodiment 1 1 H NMR and 13 C NMR) detection data a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com