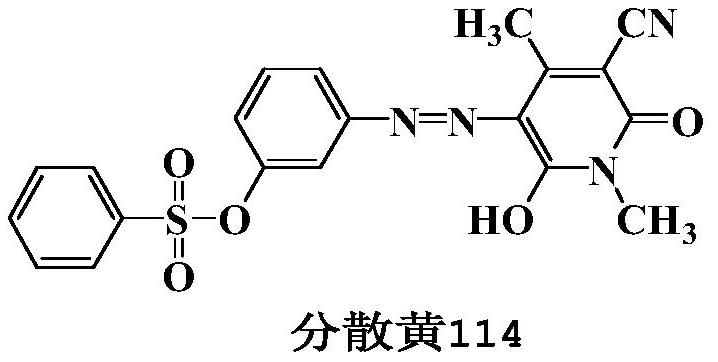
Pyridone-type azo disperse dyes containing sulfonate groups and synthesis method thereof
A pyridone-based azo and disperse dye technology, applied in azo dyes, monoazo dyes, organic dyes, etc., can solve the problems of high price of m-aminophenol, many reaction steps, three waste pollution, etc., and achieve high production value , The effect of simple synthesis and excellent comprehensive performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
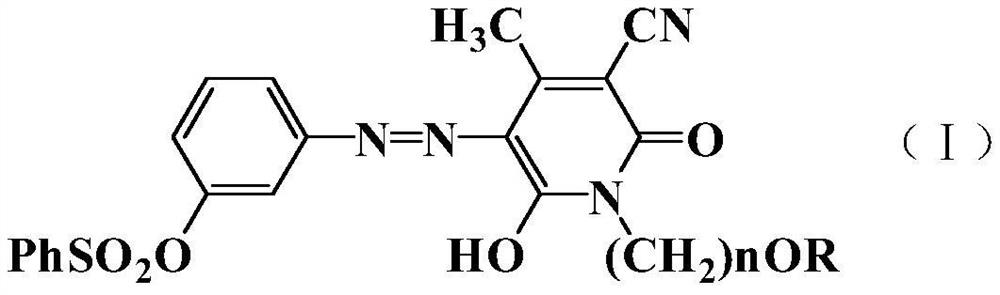
[0041] The synthesis of embodiment 1 compound 3
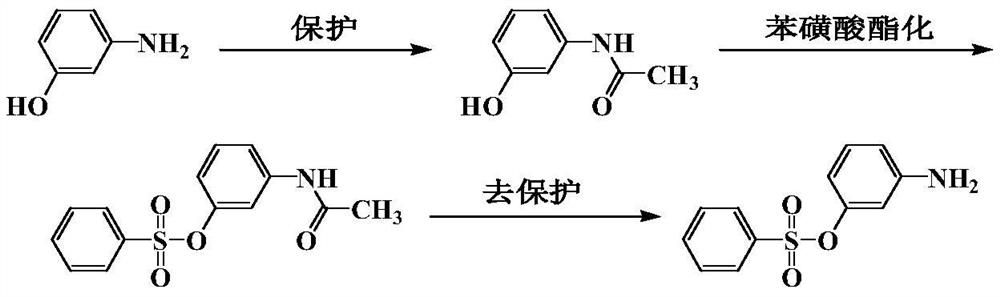
[0042] Coupling of 3-Benzenesulfonyloxyaniline with 3-cyano-4-methyl-6-hydroxy-1-(2-propoxyethyl)-2-pyridone
[0043] Add 180mL hydrochloric acid and 99.6g (0.4mol) 3-benzenesulfonyloxyaniline into a 1000mL four-necked bottle, stir for 30min, cool to below 8°C, keep adding sodium nitrite solution dropwise at this temperature, and keep warm for 3h , detect the end point, filter, and reserve. Another 96.8g (0.41mol) of 3-cyano-4-methyl-6-hydroxy-1-(2-propoxyethyl)-2-pyridone was added to water, and liquid caustic soda was added and stirred to completely dissolve, 10 Below ℃, add diazo solution dropwise to control pH around 5. Incubate at 5°C for 1 hour, at 10°C for 1 hour, then at 60°C for 2 hours, filter, wash with hot water, and drain to obtain compound 3 as a dye filter cake. The analytical results of the refined product obtained by recrystallization are as follows: MS (m / z): 497 [M+H] + , elemental analysis C 24 h 24 N ...
Embodiment 2
[0044] The synthesis of embodiment 2 compound 8
[0045] Coupling of 3-benzenesulfonyloxyaniline with 3-cyano-4-methyl-6-hydroxy-1-[2-(2-ethoxyethoxy)ethyl]-2-pyridone
[0046] Add 160mL of hydrochloric acid and 99.6g (0.4mol) of 3-benzenesulfonyloxyaniline into a 1000mL four-necked bottle, stir for 30min, cool to below 6°C, keep adding sodium nitrite solution dropwise at this temperature, and keep warm for 4h after dropping , detect the end point, filter, and reserve. Another 109.1g (0.41mol) of 3-cyano-4-methyl-6-hydroxy-1-[2-(2-ethoxyethoxy)ethyl]-2-pyridone was added to water, and liquid caustic soda was added Stir to dissolve completely, add diazo solution dropwise below 10°C, and control the pH to about 5. Incubate at 6°C for 2h, then at 80°C for 1h, filter, wash with hot water, and drain to obtain compound 8 as a dye filter cake. The analytical results of the refined product obtained by recrystallization are as follows: MS (m / z): 527 [M+H] + , elemental analysis C ...
Embodiment 3
[0047] The synthesis of embodiment 3 compound 12
[0048]Coupling of 3-benzenesulfonyloxyaniline with 3-cyano-4-methyl-6-hydroxy-1-[2-(3-propoxypropoxy)ethyl]-2-pyridone
[0049] Add 60mL of sulfuric acid, 140mL of water, and 99.6g (0.4mol) of 3-benzenesulfonyloxyaniline into a 1000mL four-necked bottle, stir for 30min, cool to below 5°C, and add sodium nitrite solution dropwise at this temperature until the end of the dropwise Incubate and react for 2 hours, detect the end point, filter, and set aside. Another 120.5g (0.41mol) 3-cyano-4-methyl-6-hydroxy-1-[2-(3-propoxypropoxy)ethyl]-2-pyridone was added to water, and liquid caustic soda was added Stir to dissolve completely, add diazo solution dropwise below 12°C, and control the pH to about 5. Incubate at 5°C for 1h, at 10°C for 1h, then at 80°C for 1h, filter, wash with hot water, and drain to obtain dye cake compound 12. The analytical results of the refined product obtained by recrystallization are as follows: MS (m / z)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com