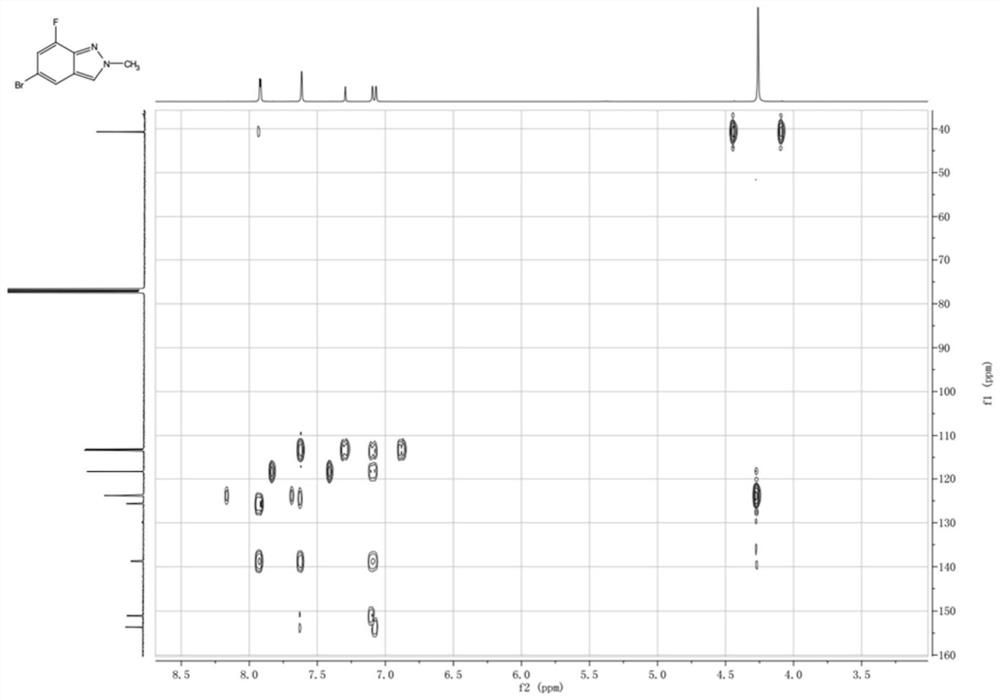
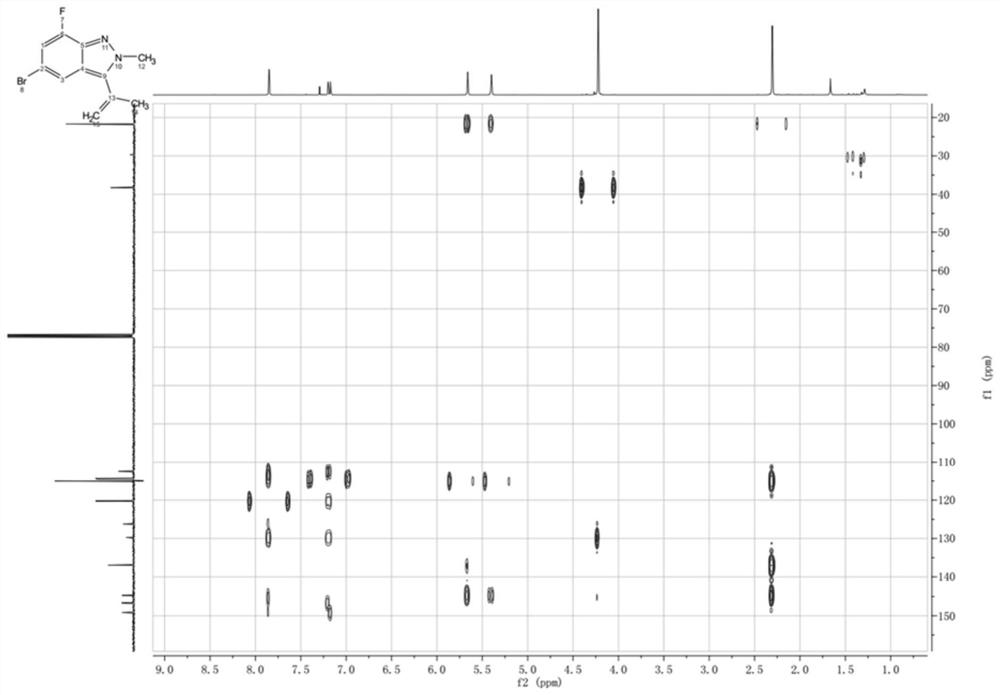
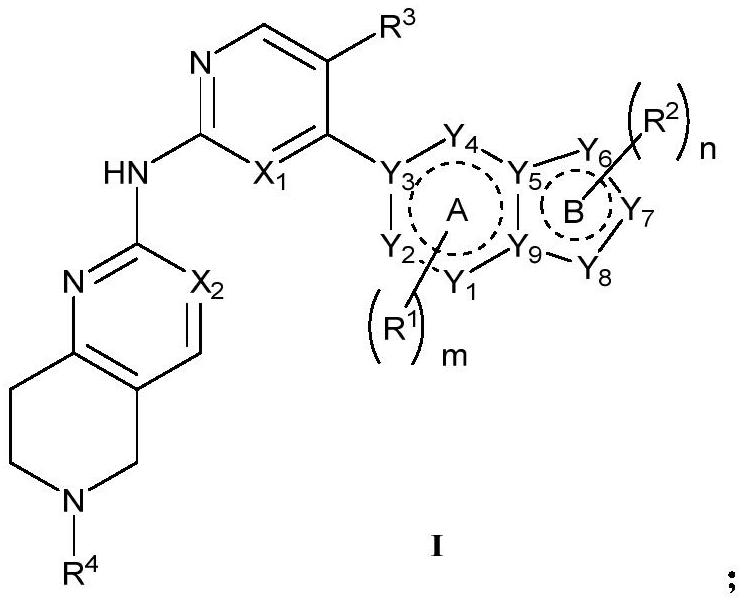
Nitrogen-containing condensed heterocyclic compound, its preparation method, intermediate, composition and application
A technology of compounds and fused heterocycles, applied in the direction of drug combinations, compounds containing elements of Group 3/13 of the periodic table, chemical instruments and methods, etc., can solve the problem of low inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0350]
[0351] first step:
[0352] Dissolve 5-bromo-1,3-difluoro-2-nitrobenzene (2.38g, 10mmol) and isopropylamine (660mg, 11mmol) in tetrahydrofuran (50ml), stir at room temperature for 16 hours, filter, and concentrate the filtrate The compound 5-bromo-3-fluoro-N-isopropyl-2-nitroaniline (2.3 g, 8.3 mmol) represented by formula 1-b was obtained. LC-MS: m / z: (M+H) + = 277.1, 279.1.
[0353] Step two:
[0354] 5-Bromo-3-fluoro-N-isopropyl-2-nitroaniline (2.3g, 8.3mmol) (compound shown in formula 1-b) was dissolved in methanol (30ml), and Raney nickel was added (500mg, 8.5mmol), then slowly dropwise added hydrazine hydrate (1.5ml), stirred at room temperature for 16 hours, filtered, and the filtrate was concentrated to give the compound 5-bromo-3-fluoro-N1-isopropyl- Phenyl-1,2-diamine (1.8 g, 7.3 mmol). LC-MS: m / z: (M+H) + = 247.1, 249.1.
[0355] third step:
[0356] Dissolve 5-bromo-3-fluoro-N1-isopropyl-phenyl-1,2-diamine (1.8g, 7.3mmol) (compound shown in form...
preparation example 2
[0358]
[0359] first step:
[0360] 5-Bromobenzo[d]oxazol-2(3H)-one (500mg, 2.3362mmol) (compound as shown in formula 2-a), 2-iodopropane (0.8g, 4.7mmol) and potassium carbonate (1g, 7.25mmol) was added to 4ml of N,N-dimethylformamide and stirred overnight at room temperature. The reaction solution was filtered, and the obtained filtrate was concentrated to obtain a crude product. After passing through the column (ethyl acetate:petroleum ether=0-10%), 520 mg of the target product (compound shown in formula 2-b) was obtained as a white solid, with a yield of 87%. LC-MS: m / z: (M+H) + =256. 1 H NMR (400MHz, CDCl 3 )δ7.25(td, J=4.3,1.8Hz,2H),7.14–7.05(m,1H),4.54(dt,J=13.9,7.0Hz,1H),1.56(d,J=7.0Hz,6H ).
[0361] Step two:
[0362] 5-bromo-3-isopropylbenzo[d]oxazol-2(3H)-one (260mg, 1.015mmol) (compound as shown in formula 2-b), double pinacol borate ( 292mg, 1.15mmol), Pd(dppf)Cl 2 .CH 2 Cl 2 (82mg, 0.1mmol) and potassium acetate (200mg, 2.04mmol) were added to 5ml o...
preparation example 3
[0368]
[0369] first step:
[0370] Compound 5-bromo-2-cyanopyridine (1.4g, 7.7mmol) as shown in formula 3-a was dissolved in tetrahydrofuran (20ml), slowly added borane (276mg, 3equiv., 23mmol), then stirred at room temperature for about 16 hours, quenched with water, stirred at 60°C for about 20 minutes, added 2N hydrochloric acid (10ml) and continued to stir for about 20 minutes, cooled to room temperature, added 2N sodium hydroxide solution to adjust the pH value to 8, concentrated to remove water, and the solid was washed with methanol / The mixed solution of dichloromethane=10 / 1 was dissolved, filtered, and concentrated to give 5-bromo-2-aminomethylpyridine (1.4g, 7.5mmol, 98% yield) as shown in the formula 3-b compound, yellow oil , used directly in the next step without purification. LC-MS: M / Z=187, 189(M+H) +
[0371] Step two:
[0372] Compound 5-bromo-2-aminomethylpyridine (1.4g, 7.5mmol) as shown in formula 3-b was dissolved in methylene chloride (20ml), the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com