Chimeric antigen receptor modified lymphocyte capable of expressing CXCR4, and preparation method and applications thereof
A chimeric antigen receptor and lymphocyte technology, applied in the field of cells, can solve the problems of limited chemotactic activity, decreased expression of chemokine receptors, and lack of tumor-specific antigens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
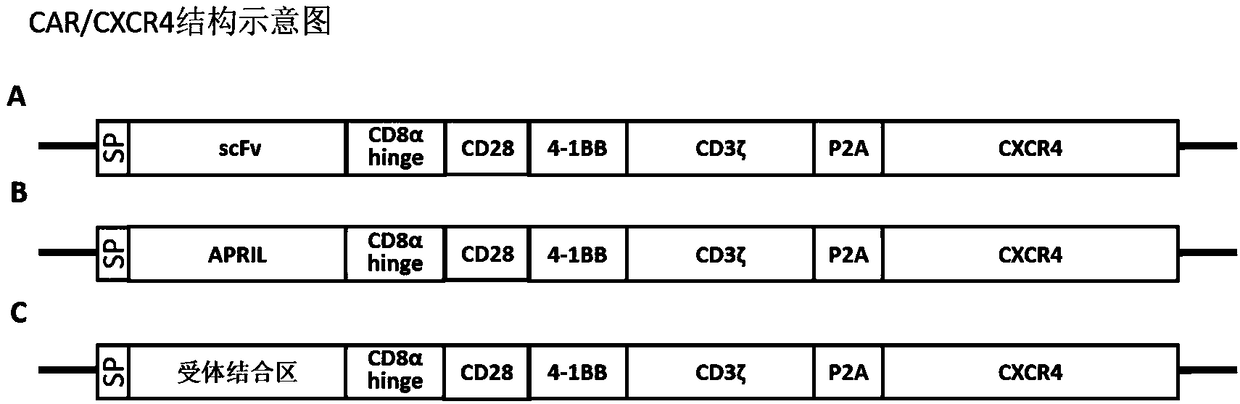
[0123] Example 1 Obtaining CAR, CAR / CXCR4 full-length genes, and completing the construction of recombinant plasmid vectors
[0124] 1. Acquisition of full-length scFv-CAR and scFv-CAR / CXCR4 gene
[0125] The scFv-CAR and scFv-CAR-P2A-CXCR4 gene fragments were obtained by the whole gene synthesis method.
[0126] At the same time, the following primers were designed to amplify the scFv-CAR fragment:
[0127] 5' Primer: 5'AGGTTTAAACTACGGATGGTGCTGCTGGTGACCTCCC3' SEQ ID NO: 25
[0128] 3' primer: 5' ATGACTAGTCCCGGGTTAGCGAGGGGGCAGGGCCTGC3' SEQ ID NO: 26
[0129] The reaction conditions are as follows:
[0130] PCR reaction: Denaturation at 94°C for 30 seconds; annealing at 60°C for 30 seconds; extension at 68°C for 1 minute. React 25 cycles. This was then extended for an additional 10 minutes at 72°C.
[0131] Design the following primers to amplify the scFv-CAR / CXCR4 fragment:
[0132] 5' Primer: 5'AGGTTTAAACTACGGATGGTGCTGCTGGTGACCTCCC3' SEQ ID NO: 27
[0133] 3' primer: ...
Embodiment 2
[0155] Example 2 Lentiviral Packaging and Production of CAR-T Cells
[0156] 1. Lentiviral packaging
[0157] 293T cells were cultured with DMEM+10% fetal bovine serum (FBS), and used for virus packaging when they grew to a density of 70-90%. The steps were as follows: 2 hours before transfection, replace with fresh preheated DMEM medium (containing 10% FBS) , Prepare the transfection system in a 15ml centrifuge tube: the experimental conditions are shown in Tables 2 and 3.
[0159]
[0160] Table 3 transfection system:
[0161]
[0162] After mixing, evenly add the transfection solution to the plate, at 37°C, 5% CO 2 Continue to grow in the incubator. After 12h, replace with DMEM+2%FBS medium. Collect the virus supernatant at 48h and 72h respectively, filter the virus liquid through a 0.45μm filter membrane, ultracentrifuge at 160,000g at 4°C for 90min, resuspend in PBS, aliquot and store at -80°C.
[0163] 2. T cell infection
[0...
Embodiment 3
[0165] Example 3 Detection of CXCR4 expression in T cells
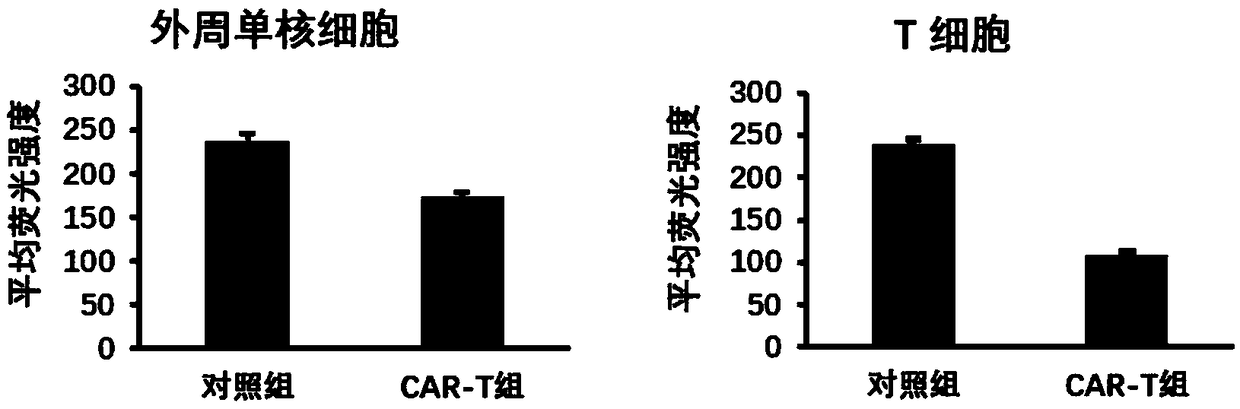
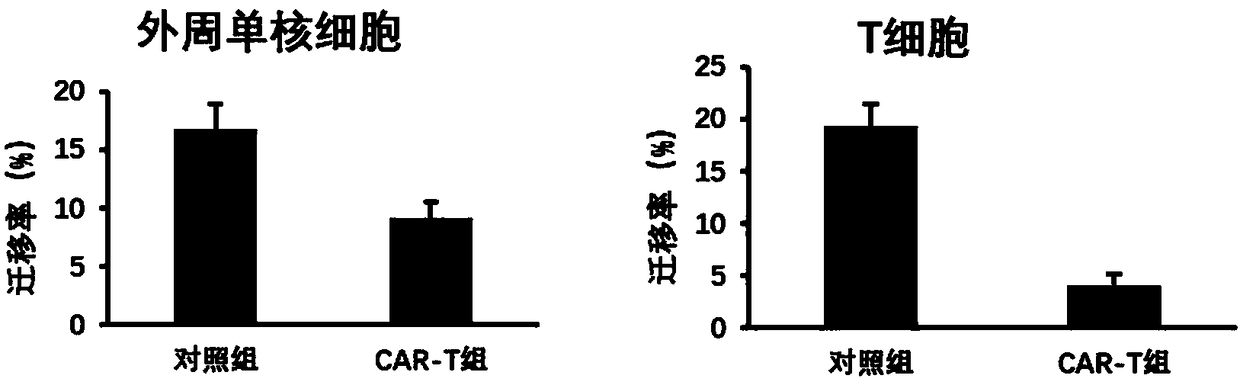
[0166] The lentivirus constructed in Example 1 was used to infect or uninfect T cells and peripheral blood mononuclear cells, change the medium after 24h, and use X-VIVO 15 (04-418Q, LONZA company) complete medium+10% human AB serum ( H4522, sigma company) continued to culture for 5 days, and at the same time, 100 U / ml of recombinant human interleukin 2 (IL-2) cytokine (C013, novaprotein company) was added. Then the cells were collected, washed twice with PBS, stained with APC-labeled anti-human CXCR4 antibody (product number 306510, biolegend company), and the expression of CXCR4 in the cell membrane was detected by flow cytometry. The expression of CXCR4 in blood monocytes decreased significantly, and its fluorescence intensity (log value) decreased from 238 to 174 ( Figure 4 ), the expression of CXCR4 decreased more significantly after T cells were infected with lentivirus, and its fluorescence intensity (log val...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com