A kind of nanoparticle and its preparation method and application
A nanoparticle and small interference technology, applied in the field of medicine, can solve the problems of high biological toxicity, difficulty in large-scale production, high production cost, etc., and achieve the effect of enhancing the inhibitory effect and promoting the proliferation and activation of T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
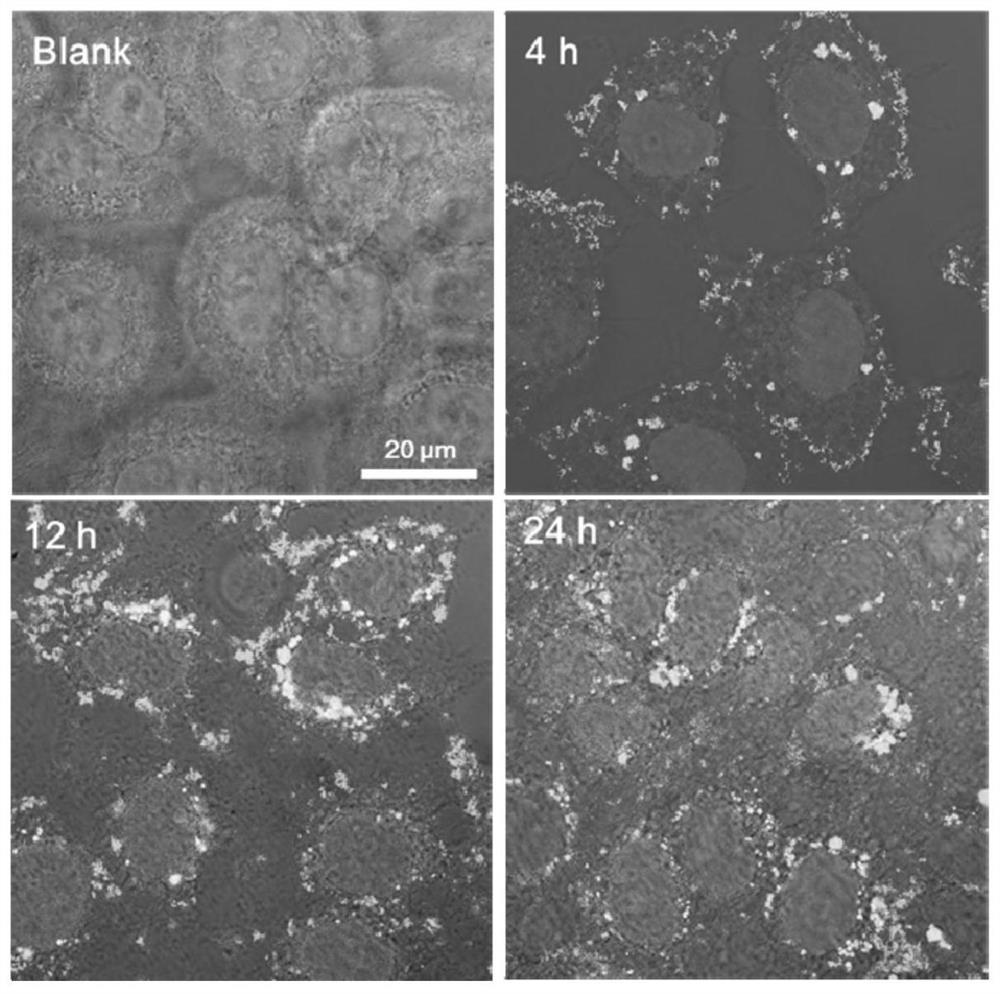
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of nanoparticle vaccine
[0050] Preparation of LDH: quickly add 10mL magnesium nitrate (0.03M) and aluminum nitrate (0.01M) mixture into 40mL NaOH (0.15M) solution, stir rapidly at room temperature for 15min, then centrifuge at 12000g for 5min. After the obtained product was resuspended in decarbonated water, it was shaken and suspended at 30°C for 30 min, and the resulting suspension was transferred to a dialysis bag, and dialyzed in a closed container with decarbonated water 100 times the volume of the suspension at room temperature for 5 h. Then the suspension was transferred to an airtight container, shaken and suspended at 30°C for 1h at 280rpm, then transferred to a 100mL polytetrafluoroethylene hydrothermal kettle (50% sample volume), kept at 120°C for 15h, and cooled naturally.
[0051] Preparation of LDH / IDO-siRNA: Mix the prepared hydrotalcite with IDO siRNA, the sense strand (SEQ ID NO.1) of the IDO siRNA: 5'-GGGCUUCUUCCUCGUCUCU...
Embodiment 2
[0055] Example 2: Determination of the ability of IDO-siRNA to be inserted into LDH in vaccines
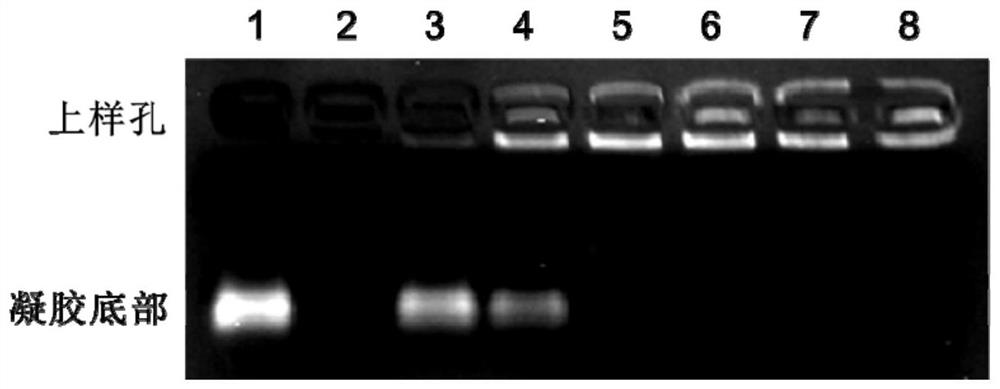
[0056] Agarose gel electrophoresis was performed according to conventional methods. In the gel electrophoresis diagram, free IDO siRNA migrated to the bottom of the gel, and IDO siRNA that entered the LDH layer remained in the sample well. The calculation method for the ability of IDO-siRNA intercalation into the LDH interlayer is as follows:
[0057] The IDO siRNA intercalation ability of hydrotalcite is calculated according to the following formula:
[0058]
[0059] Test results such as figure 1 As shown, the mass of free IDO siRNA in channel 1 is 1 μg, the mass of LDH in channel 2 is 40 μg, the mass of IDO siRNA in channels 3-8 is 1 μg, and the mass of LDH is added according to different mass ratios.
[0060]The results show that using the method described in the present invention to intercalate IDO siRNA to LDH, when the mass ratio of hydrotalcite and IDOsiRNA is 10:1, I...
Embodiment 3
[0061] Example 3: Determination of the ability of LDH / IDO-siRNA to adsorb Trp2
[0062] The conventional 2,2-biquinoline-4,4-dicarboxylate disodium assay method was used to determine the ability of LDH / IDO-siRNA to adsorb Trp2, and the adsorption capacity of LDH / IDO-siRNA to Trp2 was calculated according to the following formula:
[0063]
[0064] Description: Mg 3 The quality of Al-IDO siRNA-LDH is based on the mass of hydrotalcite
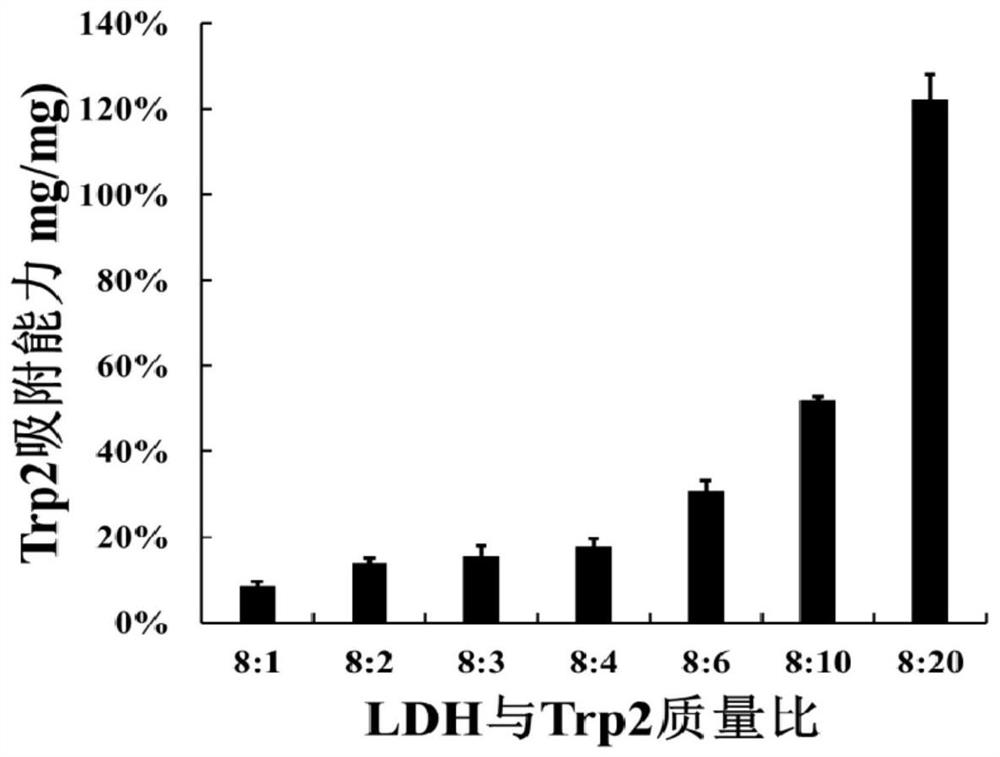
[0065] Test results such as figure 2 Shown:
[0066] When the mass of LDH / IDO-siRNA and Trp2 is 8:1, the adsorption capacity of Trp2 is 8.5%;
[0067] When the mass of LDH / IDO-siRNA and Trp2 is 8:2, the adsorption capacity of Trp2 is 13.9%;
[0068] When the mass of LDH / IDO-siRNA and Trp2 is 8:3, the adsorption capacity of Trp2 is 15.5%;
[0069] When the mass of LDH / IDO-siRNA and Trp2 is 8:4, the adsorption capacity of Trp2 is 17.8%;
[0070] When the mass of LDH / IDO-siRNA and Trp2 is 8:6, the adsorption capacity of Trp2 is 30.9%;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com