System, apparatus and method of assembly for administering substances
A kind of material and remote technology, applied in the direction of drug equipment, medical science, surgery, etc., can solve the problems that have not promoted rapid GI tract drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Proof of Concept for Ultrasound for In Vitro Drug Delivery
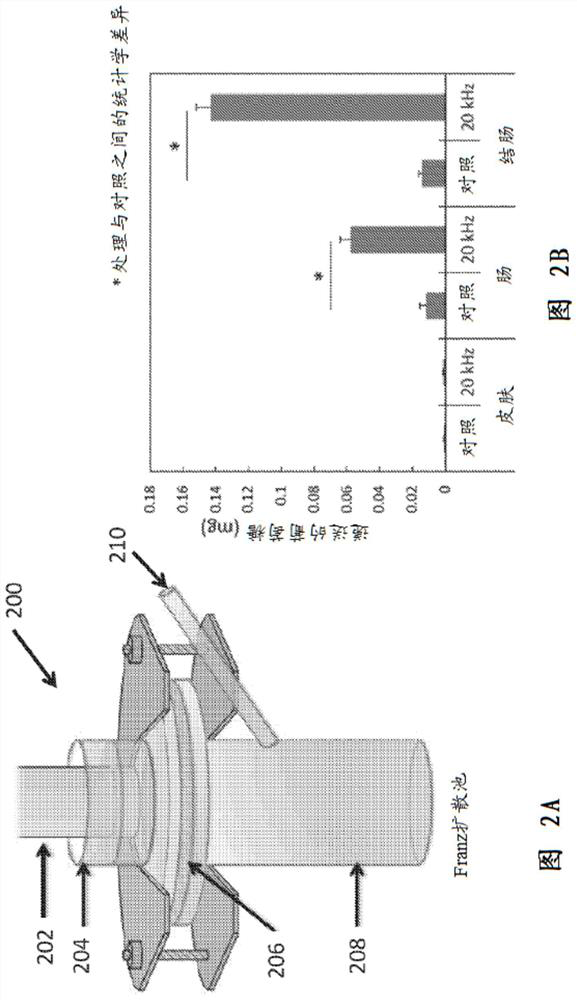
[0070]To understand whether ultrasound can safely penetrate GI tissue to allow enhanced drug delivery and to determine optimal conditions for UMGID, an in vitro platform was developed using fresh porcine GI tissue mounted in a Franz diffusion cell (see Figure 2A ). The focus was on the use of low frequency (less than 100 kHz) ultrasound in view of previous data showing increased cavitation activity at common intensities compared to high frequency (greater than 1 MHz) ultrasound at the same intensities.
[0071] A. Experimental setup
[0072] Phosphate buffered saline (PBS), hydrocortisone, ammonia salicylic acid, dahlia inulin (5,000 Da) and deuterated dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (Saint Louis, Missouri). Granular D-glucose was obtained from Mallinckrodt Chemicals (Phillipsburg, New Jersey). Lysine labeled with Texas Red was purchased from Invitrogen (Carlsbad, Cali...
Embodiment 2
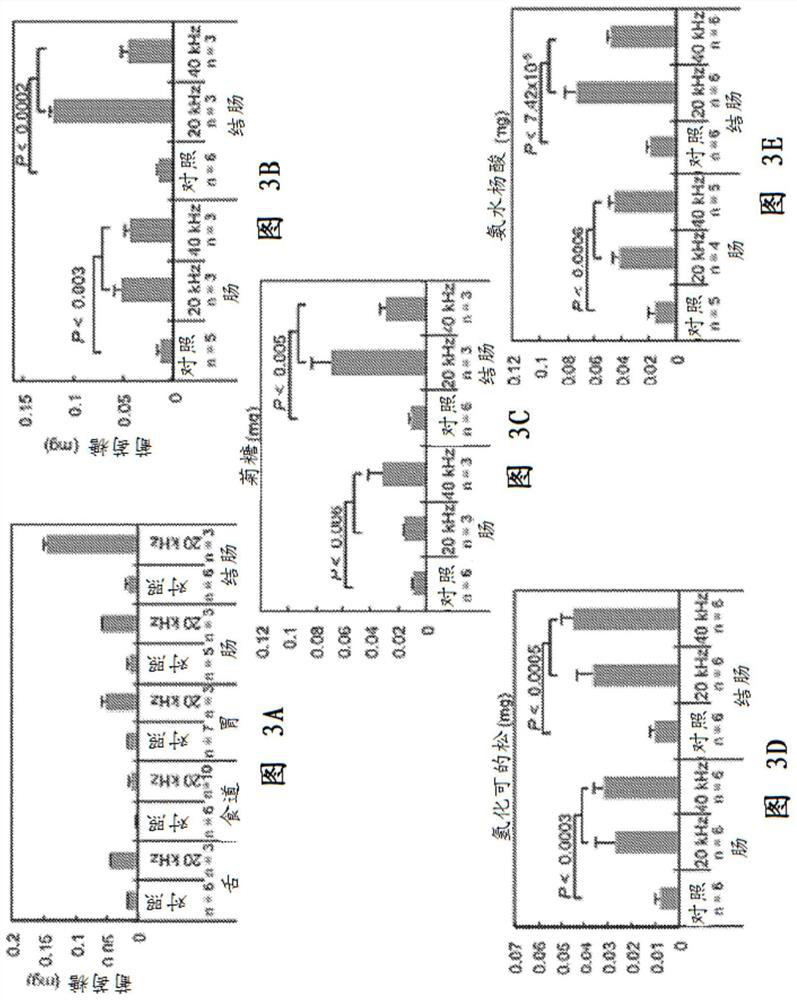
[0090] Example 2: Characterization of the underlying mechanism of delivery enhancement
[0091] Based on previous studies evaluating phenomena associated with ultrasonic penetration of liquids, the observed enhancement can be attributed to one or more mechanisms including, but not limited to: (1) acoustic fluidization, (2) thermal effects, and (3) ) transient cavitation. To elucidate which mechanisms predominate, tritium was evaluated under the separation effect of stirring the donor chamber (to mimic general stirring that reduces the diffusion boundary layer) and sonication using 1 MHz ultrasound at an intensity below the transient cavitation threshold delivery of glucose to the small intestine to isolate the effects of acoustic fluidization and tissue heating. These protocols were compared to delivery using 20 and 40 kHz ultrasound.
[0092] A. Acoustic Fluidization
[0093] To investigate the effects of acoustic fluidization and agitation, tissue samples were mounted in ...
Embodiment 3
[0134] Example 3: Effects of sonication on the structure and function of therapeutic compounds
[0135] The effect of sonication on the molecular structure of ammonia salicylic acid and hydrocortisone was investigated by analyzing the sonicated molecules with nuclear magnetic resonance (NMR). Deuterated DMSO solutions of ammonia salicylic acid and hydrocortisone were prepared at a concentration of 4 mg / mL. A 1.5 mL sample was sonicated as described above with 20 kHz ultrasound at the highest intensity considered. Three biological parallels were performed. Samples without sonication were used as controls. A Varian 500 (1H, 500 MHz) spectrometer was used to record 1H NMR spectra followed by Mnova NMR software (from Mestralab Research, A Spain) processing. The 1H NMR spectrum (DMSO-d5=2.5 ppm) is quoted in conjunction with the residual non-deuterated solvent offset. All offsets are reported in ppm. The volatile tetramethylsilane (TMS) internal standard was found to disappe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com