Molecular machines
A technology of cofactors, complexes, applied in chemical instruments and methods, immobilized on/in organic carriers, enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
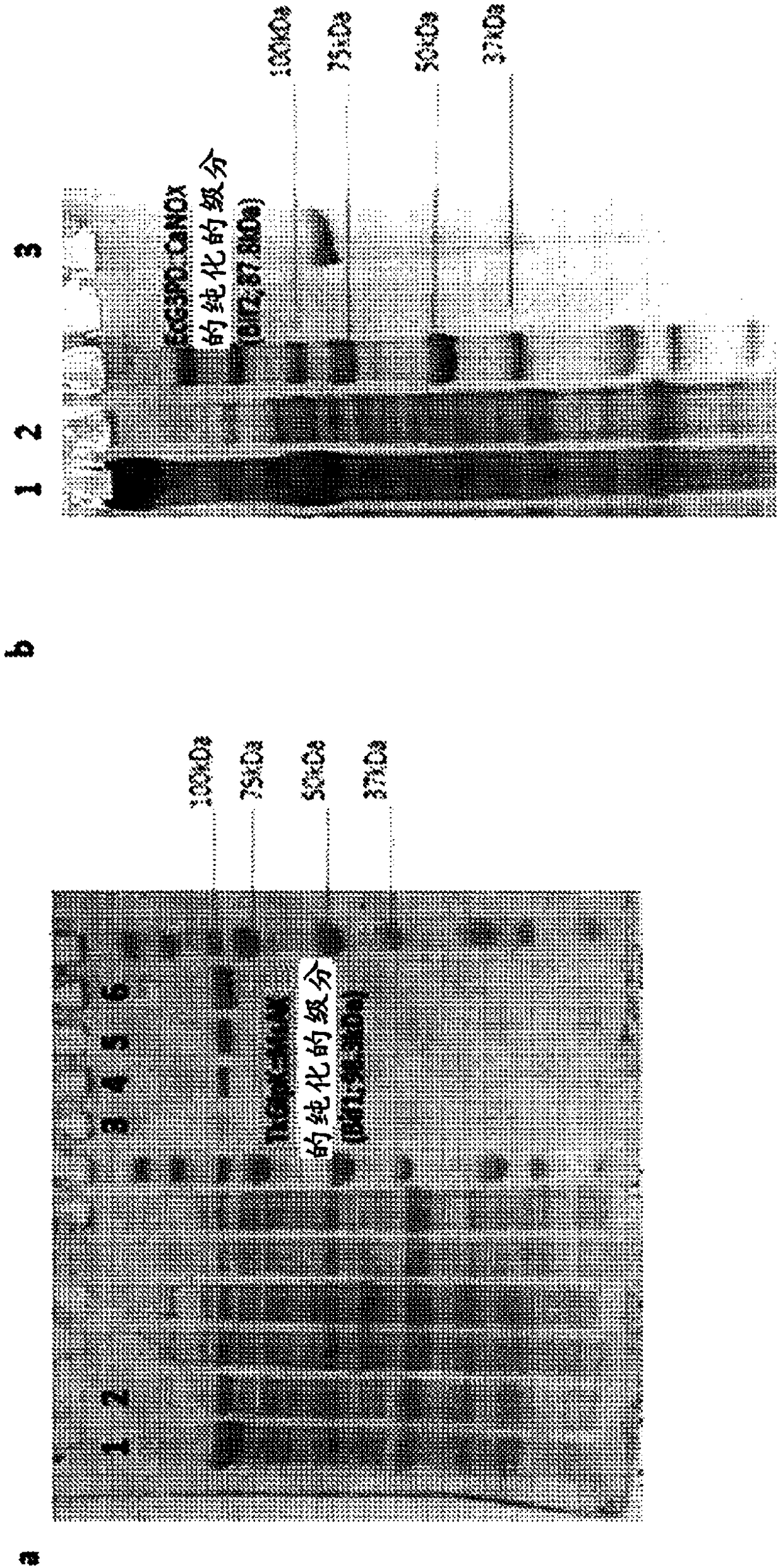
[0297] Example 1 - Construction and demonstration of a dual-enzyme fusion protein
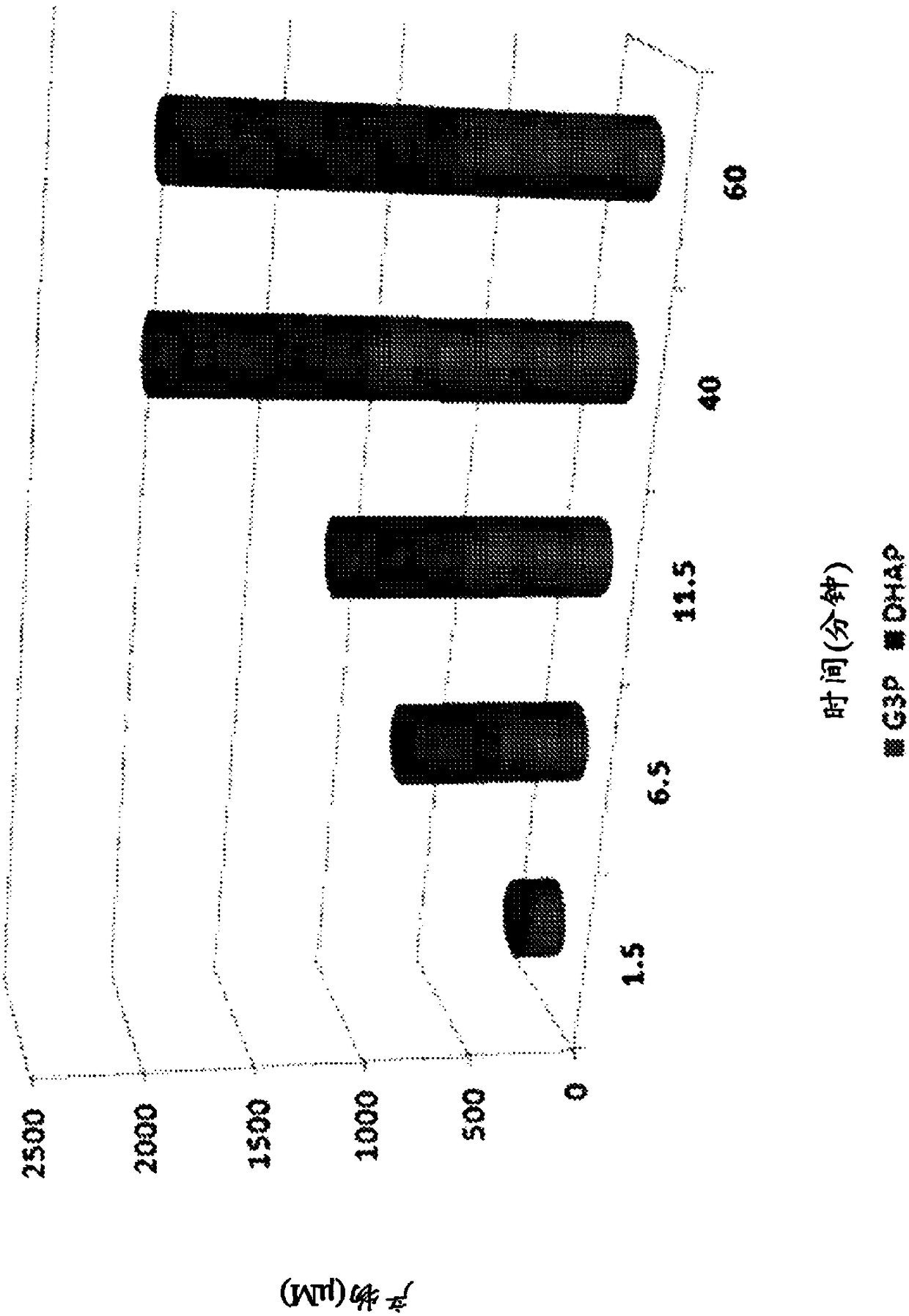
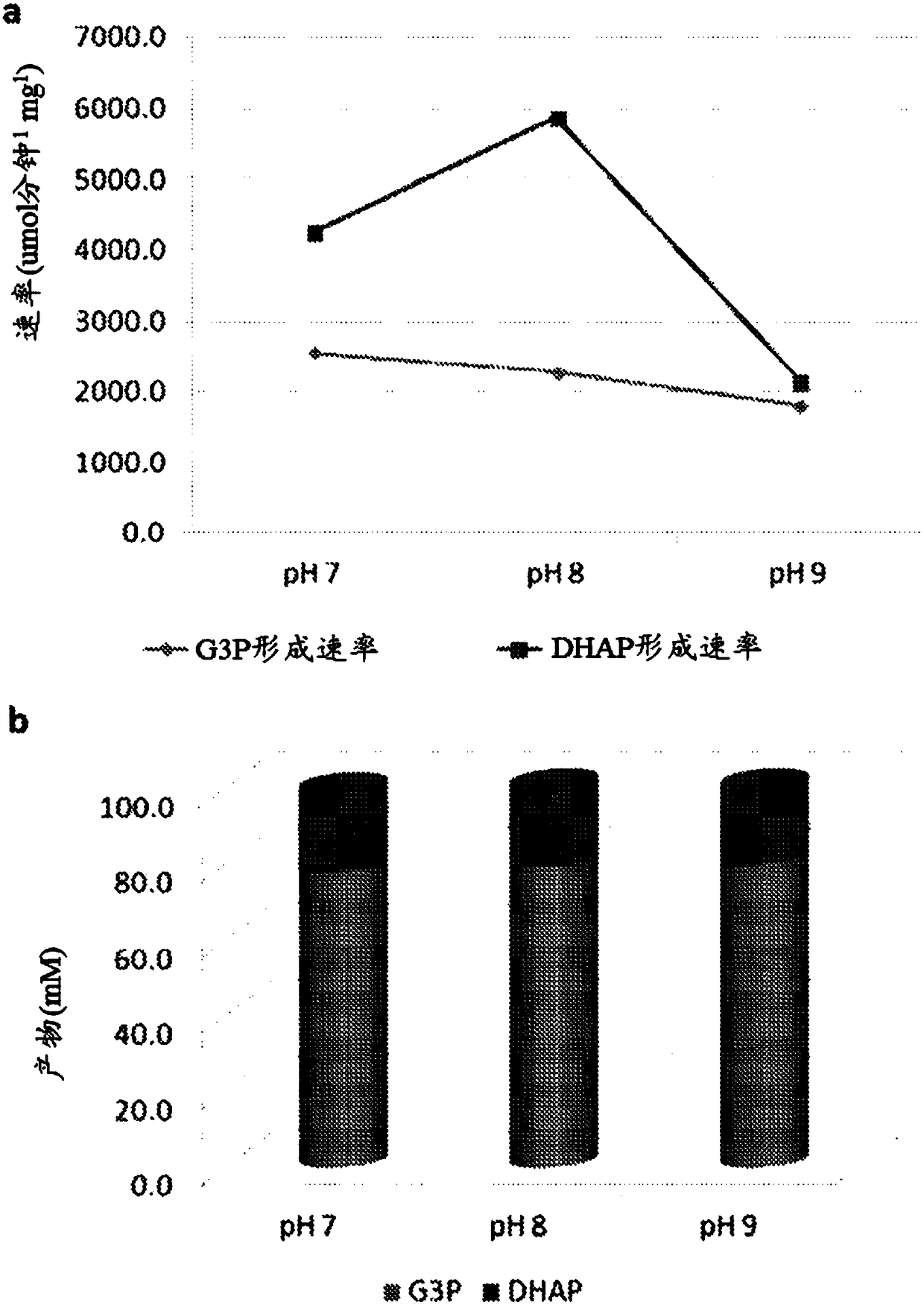
[0298] 22 enzymes were evaluated for the synthesis steps of DHAP from glycerol (region-specific phosphorylation and oxidation) and proper cofactor recycling. The four best enzyme combinations were then used to synthesize dual-enzyme fusion proteins. Each fusion protein produced is a single molecule encoding two functionalities (DHAP synthesis step and cognate cofactor recycling).
[0299] By combining the gene encoding the related enzyme with the encoding containing GlySerSer repeat sequence (GSS) n (GSS) 3 C(GSS) 3 ) to generate a dual-enzyme fusion protein.
[0300] Bienzymatic Fusion 1 (BiF1) contains the optimal enzymes for glycerol-3-phosphate production and ATP regeneration (Thermococcus Kagoshima glycerol kinase [TkGlpK] and Mycobacterium smegmatis ATP kinase [MsAK]).
[0301] Bienzymatic Fusion 2 (BiF2) contains the optimal enzymes to generate DHAP from glycerol-3-phosphate and r...
Embodiment 3
[0412] Example 3 - Nanofactory Comprising Three Nanomachine Flow Reactors
[0413] Preparation of Sepharose Beads with Immobilized 1,1,1-Trifluoro-3-((6-Mercaptohexyl)thio)propan-2-one (TFK)
[0414] Add saturated NaHCO to a slurry of vinylsulfone-activated agarose (800 mL, 600-800 mmol vinylsulfone groups, 50% slurry in 1:1 ethanol / water) 3 Aqueous solution (80 mL), 1,1,1-trifluoro-3-((6-mercaptohexyl)thio)propan-2-one (104 mg, 0.4 mmol) dissolved in ethanol (4.8 mL). The mixture was stirred slowly overnight at room temperature. Excess reaction sites were blocked by addition of 2-mercaptoethanol (11.2 mL, 80 mmol), and stirring was continued for 6 hours. The resin was then thoroughly washed with 50% ethanol / water until no noticeable odor. Beads were stored as a 1:1 slurry in 50% ethanol / water.
[0415] Triple multienzyme reactor using fusion enzymes immobilized on TFK-derived agarose beads
[0416] TriF2 with tethered mNAD (EcG3PD-CaNOX-AaE2), galactose oxidase M3-5 -...
Embodiment 4
[0431] Example 4 - Biocatalytic Flow Reactor
[0432] D-buckwheat alkali nanofactory
[0433] The functionality of immobilized nanomachines in a reactor that retains and recycles cofactors for flow biocatalysis was demonstrated via the production of the important commercially relevant antidiabetic drug D-buckwine. D-buckwine can be enzymatically converted from glycerol via two regiospecific cofactor-dependent steps (ATP-dependent phosphorylation and NAD-dependent oxidation) and stereospecific aldol condensation) followed by chemical cyclization. produce( Figure 36 ).
[0434] phosphorylation transfer reactor
[0435] In order to prepare the TriF1 phosphotransfer reactor ( Figure 36 In step 1), 40 mg of TriF1 protein (296 nmol) was immobilized on 25 g of Sepharose-Hexyl-DVS-TFK beads. Immobilized TriF1 was treated with TCEP, washed with degassed spray PBS containing 0.5 mM EDTA, and then incubated at 4 °C with 6 equivalents of ADP-2AE-PEG 24 -NAD was reacted for 6 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com