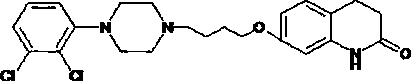
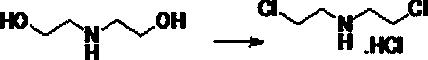Preparation method of 2,3-dichlorophenylpiperazine hydrochloride
A technology of dichlorophenylpiperazine and hydrochloride, applied in 2 fields, can solve the problems of high raw material cost of aripiprazole, complicated operation steps and the like, and achieve the effects of high product yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] In a typical embodiment of this application, a method for preparing 2,3-dichlorophenylpiperazine hydrochloride is proposed, and the method includes:
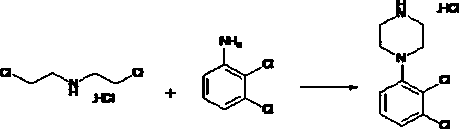
[0026] (A) Cyclization reaction of bis(2-chloroethyl)amine hydrochloride and 2,3-dichloroaniline in N,N-dimethylformamide single solvent to obtain 2,3-dichlorophenyl Piperazine hydrochloride step.
[0027] In another typical embodiment of the present application, a method for preparing 2,3-dichlorophenylpiperazine hydrochloride is proposed, and the method includes:
[0028] (A1) Diethanolamine is used as raw material to generate bis(2-chloroethyl)amine hydrochloride through chlorination reaction;
[0029] (A2) Cyclization reaction of bis(2-chloroethyl)amine hydrochloride and 2,3-dichloroaniline in N,N-dimethylformamide single solvent to obtain 2,3-dichlorophenyl Piperazine hydrochloride step.
[0030] In the technical scheme of the present invention, a single N,N-dimethylformamide solvent is used, so that the 2,3-dichlorophenylpi...
Embodiment 1
[0032] (1) Preparation of bis(2-chloroethyl)amine hydrochloride
[0033]
[0034] Weigh 200g of diethanolamine and add 800ml of toluene into a 2L three-necked flask, stir to dissolve, keep the temperature at 20-30°C, add 566g of thionyl chloride dropwise, heat up to 50°C, keep it for 3h and evaporate the solvent under reduced pressure to recover toluene.
[0035] Add 100ml of toluene to the remaining residue, stir at 20-30°C for 30min, filter, and dry to obtain 322g of bis(2-chloroethyl)amine hydrochloride as a white solid, yield: 94.7%.
[0036] (2) Preparation of 2,3-dichlorophenylpiperazine hydrochloride
[0037]
[0038] Weigh 130g of 2,3-dichloroaniline into a 500ml three-necked flask, add 144g of bis(2-chloroethyl)amine hydrochloride, then add 200ml of DMF (N,N-dimethylformamide), and stir for 5min. , Heated to 140-145°C, reacted for 6 hours, HPLC monitored the reaction to complete, and then cooled to 5-10°C to obtain 200 g of light yellow solid. The above solid is dissolved in...
Embodiment 2
[0042] (1) Preparation of bis(2-chloroethyl)amine hydrochloride
[0043] Dissolve 523 g (4.4 mol) of thionyl chloride in 100 mL of chloroform, place it in a four-necked flask equipped with a reflux condenser and an ice-water bath, stir, and absorb the tail gas with water. Slowly add a mixture of 215 g (2 mol) of diethanolamine and 150 mL of chloroform. During the addition, control the temperature of the reaction solution to not exceed 30°C, and the addition time is about 3 h. After the addition is complete, remove the ice-water bath and react at room temperature for 1 h, then slowly increase the temperature. After the solid is completely dissolved, the temperature is increased to 50°C, and the reaction is continued for 0.5 h. Stop heating, cool to room temperature, and filter with suction to obtain a slightly white and yellowish green solid , Recrystallized with absolute ethanol to obtain 291g of white needle-like crystals, with a yield of 81%, and a melting point of 214~216°C. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com