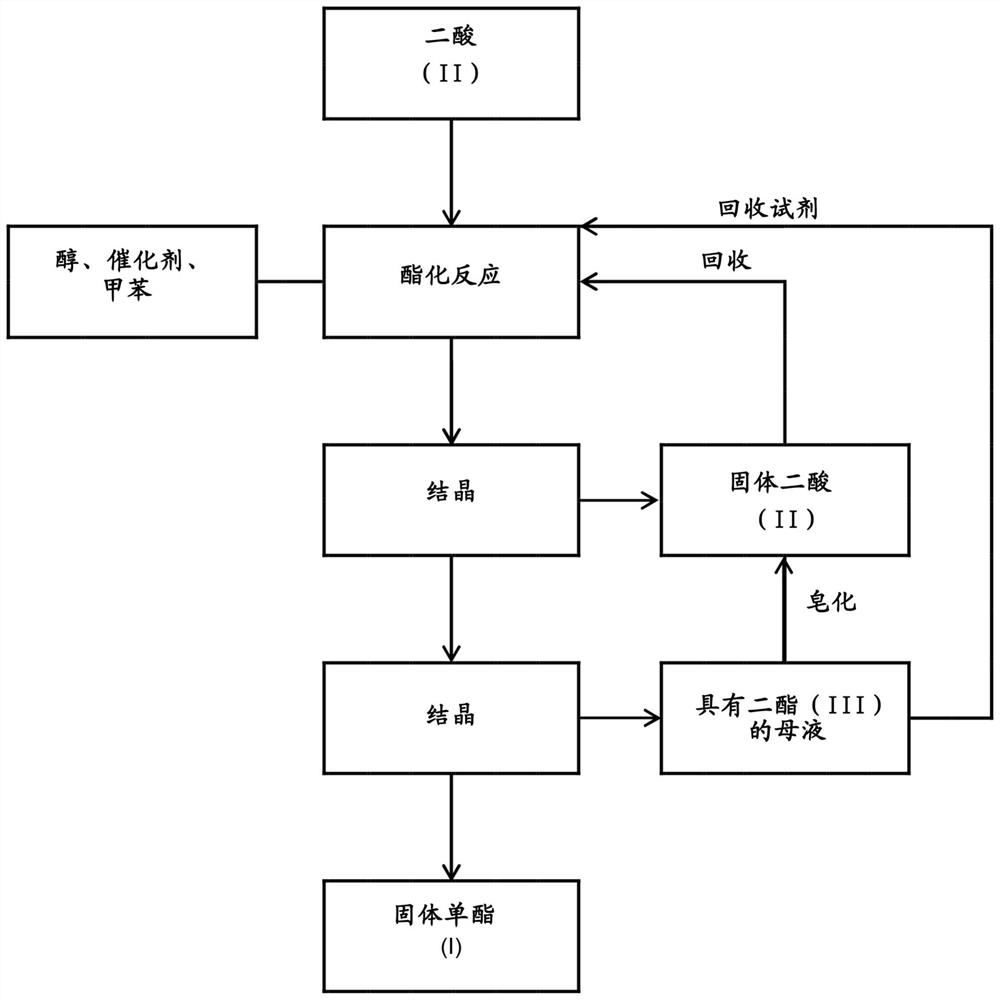
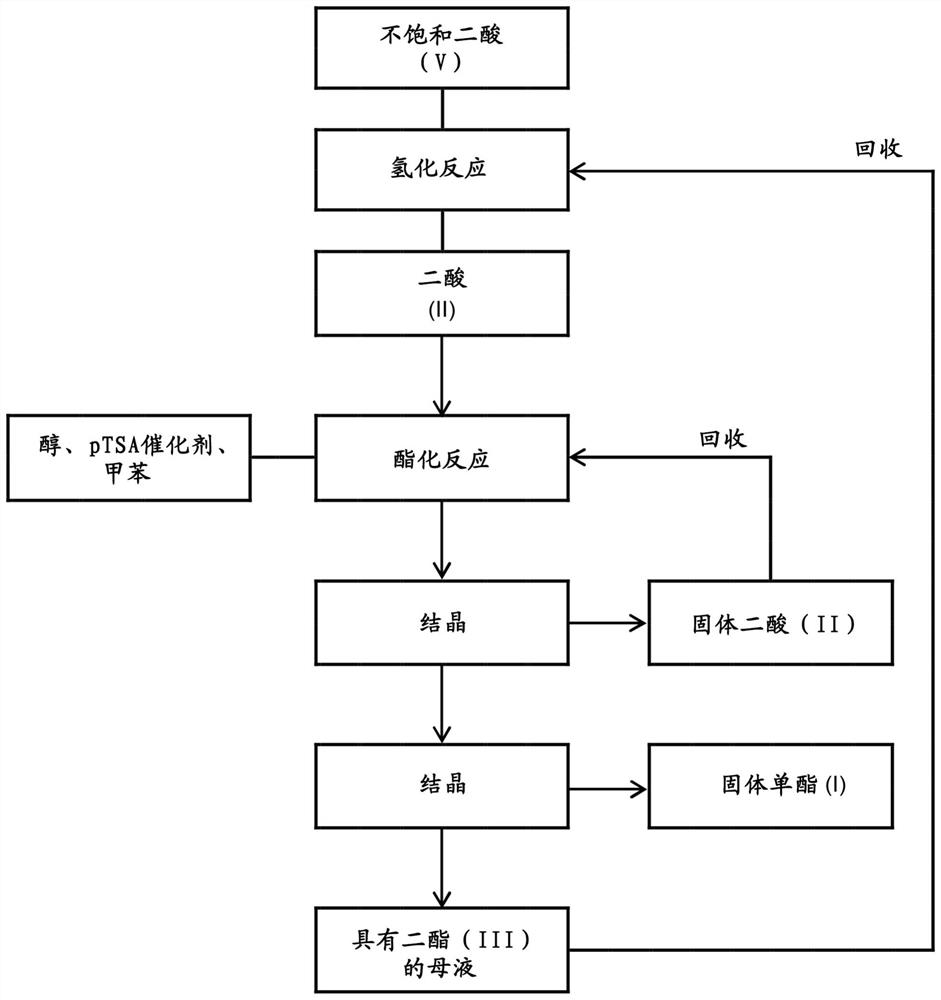
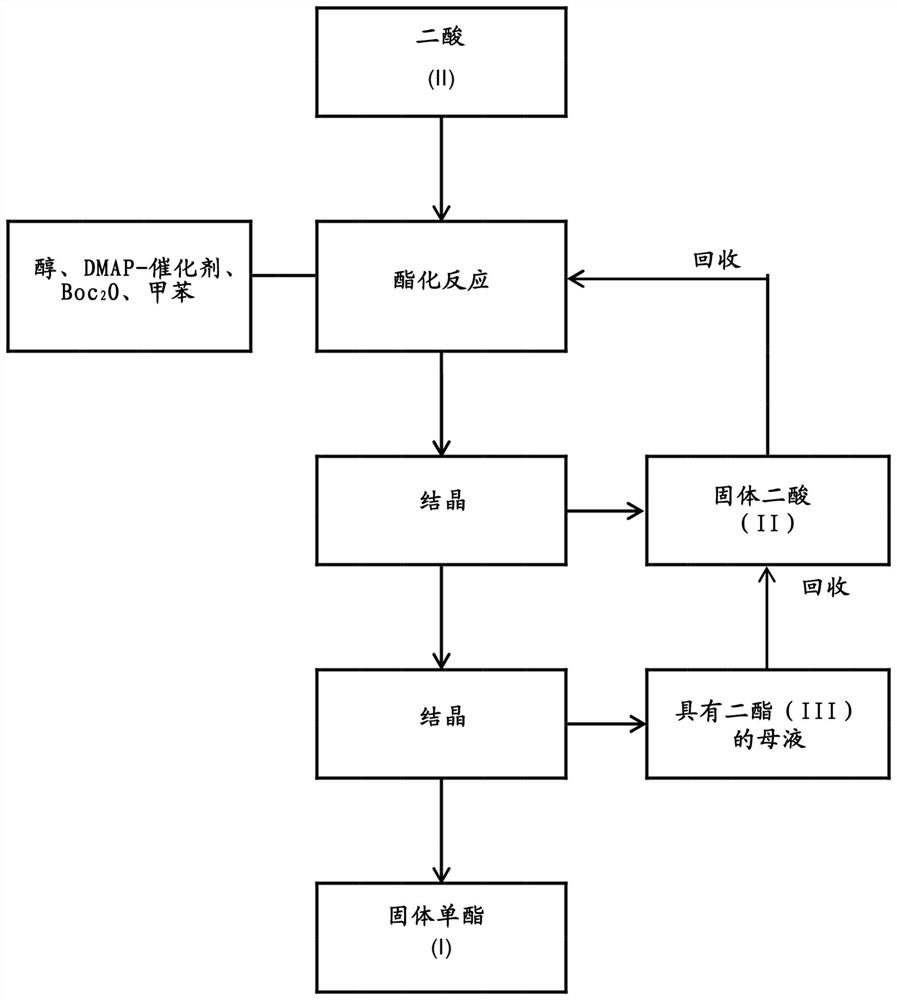
A kind of method of manufacturing long-chain diacid monoester
A technology of chain diacid monoester and diacid, which is applied in the field of synthesis of monoesterified long-chain diacids, can solve the problems of low yield of long-chain diacids, etc., and achieves the effect of protecting the environment and low-waste process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Eicosa-10-enedioic acid
[0060] An inert reactor was charged with 65 g of 10-undecenoic acid and 195 g of toluene. A solution of 100 mg of AS2053 nitro-Grela-I2SIPr (purchased from Apeiron) in 65 g of toluene was added over 5 hours at 40°C with continued removal of headspace air under a slight nitrogen purge. in a solution. The reaction was aged for 2 hours and then heated until a clear solution formed (about 80°C). Finally the solution was cooled to room temperature (about 20°C) and the precipitated product was isolated and dried. 53.0 g of the title compound were obtained. GC-MS analysis of the sample (derivatized with BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide)) showed that the product contained 97.5% of the title as a mixture of E and Z isomers Product, 1.0% nonadecenedioic acid, 0.3% 10-undecenoic acid and small amounts of other impurities (mainly homologues of the product).
Embodiment 2
[0061] Example 2: Eicosa-10-enedioic acid
[0062] An inert reactor was charged with 20 g of 10-undecenoic acid and 60 g of toluene. A solution of 40 mg of M73SIPr (purchased from Umicore) in 20 g of toluene was added to the first solution over 5 hours at 40°C with continued headspace air removal under a slight nitrogen purge. The reaction was aged for 2 hours and then heated until a clear solution formed (about 80°C). Finally the solution was cooled to room temperature (about 20°C) and the precipitated product was isolated and dried. 14 g of the title compound were obtained. GC-MS analysis of the sample (derivatized with BSTFA) showed the product to contain 97% of the title product as a mixture of E and Z isomers, 1.3% nonadecenedioic acid, 0.2% 10-undecene alkenoic acid and a small amount of other impurities (mainly homologues of the product).
Embodiment 3
[0063] Example 3: Eicosa-10-enedioic acid
[0064] An inert reactor was charged with 20 g of 10-undecenoic acid and 60 g of toluene. A solution of 40 mg of AS2086 (purchased from Apeiron Corporation) in 20 g of toluene was added to the first solution over 5 hours at 40°C with continued headspace air removal under a slight nitrogen purge. The reaction was aged for 2 hours and then heated until a clear solution formed (about 80°C). Finally the solution was cooled to room temperature (about 20°C) and the precipitated product was isolated and dried. 15 g of the title compound were obtained. GC-MS analysis of the sample (derivatized with BSTFA) showed the product to contain 98% of the title product as a mixture of E and Z isomers, 0.8% nonadecenedioic acid, 0.1% 10-undecene alkenoic acid and a small amount of other impurities (mainly homologues of the product).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com