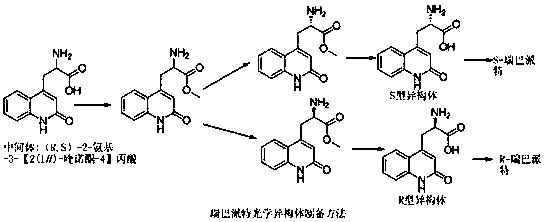
Novel synthetic method for rebamipide intermediate
A technology for rebamipide and intermediates, which is applied in the field of synthesis of rebamipide intermediates, can solve the problems of complex and tedious splitting methods, and achieves the effects of avoiding many reaction steps, avoiding splitting steps, and simple and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Rebamipide key intermediate 2-amino-3-[2(1 H )-quinolone-4] the preparation of propionic acid
[0018] Add 10g (75.1mmol) of aspartic acid L-aspartic acid, 13.1g (90.1mmol) of 2-hydroxyquinoline, and 1.3g (7.51mmol) of silver nitrate into a mixed solution of 400mL of acetonitrile and 100mL of water, and heat up to 300 mL of 0.13 g / mL potassium persulfate aqueous solution was slowly added dropwise at 70 ° C, and reacted at 70 ° C for 3 h after the drop was completed. Cool the reaction solution to room temperature, add 400 mL of purified water, extract with ethyl acetate 800 mL*3, combine the organic layers, wash with water 2000 mL*3, dry over anhydrous sodium sulfate, filter with suction, concentrate the filtrate under reduced pressure, and crystallize with acetone to obtain S- 2-Amino-3-[2(1 H )-quinolone-4]propionic acid 6.8g (29.3mmol), molar yield: 39%. HPLC content 99.60%; m.p.: 196-198 °C; [α] 20 =+21.5 。 (DMSO); 1 H-NMR (600 MHz, DMSO) δ: 11.7(bs, ...
Embodiment 2
[0019] Embodiment 2 rebamipide key intermediate 2-amino-3-[2(1 H )-quinolone-4] the preparation of propionic acid
[0020] Add 10g (75.1mmol) of aspartic acid D-aspartic acid, 12.0g (82.6mmol) of 2-hydroxyquinoline, and 1.7g (9.8mmol) of silver nitrate into a mixed solution of 400mL of acetonitrile and 100mL of water, and heat up to Slowly add 300 mL of 0.13 g / mL potassium persulfate aqueous solution dropwise at 60°C, and react at 60°C for 4 hours after the drop is completed. Cool the reaction solution to room temperature, add 400 mL of purified water, extract with 800 mL*3 of ethyl acetate, combine the organic layers, wash with 2000 mL*3 of water, dry over anhydrous sodium sulfate, filter with suction, concentrate the filtrate under reduced pressure, and crystallize with acetone to obtain R- 2-Amino-3-[2(1 H )-quinolone-4] propionic acid 6.6g (28.5mmol), molar yield: 38%. [α] 20 =-20.2 。 (DMSO); HPLC content 99.7%.
Embodiment 3
[0021] Embodiment 3 rebamipide key intermediate 2-amino-3-[2(1 H )-quinolone-4] the preparation of propionic acid
[0022] Add aspartic acid D, L-aspartic acid 10g (75.1mmol), 2-hydroxyquinoline 16.4g (112.6mmol), silver nitrate 1.3g (7.51mmol) into the mixed solution of acetonitrile 400mL and water 100mL, Raise the temperature to 80°C and slowly add 300mL of 0.13g / mL potassium persulfate aqueous solution dropwise, and react at 80°C for 3h after the dropwise completion. Cool the reaction solution to room temperature, add 400 mL of purified water, extract with 800 mL*3 of ethyl acetate, combine the organic layers, wash with 2000 mL*3 of water, dry over anhydrous sodium sulfate, filter with suction, concentrate the filtrate under reduced pressure, and crystallize with acetone to obtain racemization Body (R,S)-2-amino-3-[2(1 H )-quinolone-4]propionic acid 5.9g (25.5mmol), molar yield: 34%. HPLC content 99.53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com