Method for preparing modified SnO2 electrode and application of method for preparing formic acid by photoelectric catalytic reduction of CO2
A photoelectric catalysis, CO2 technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problems of low utilization rate of visible light, high reaction overpotential, poor product selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
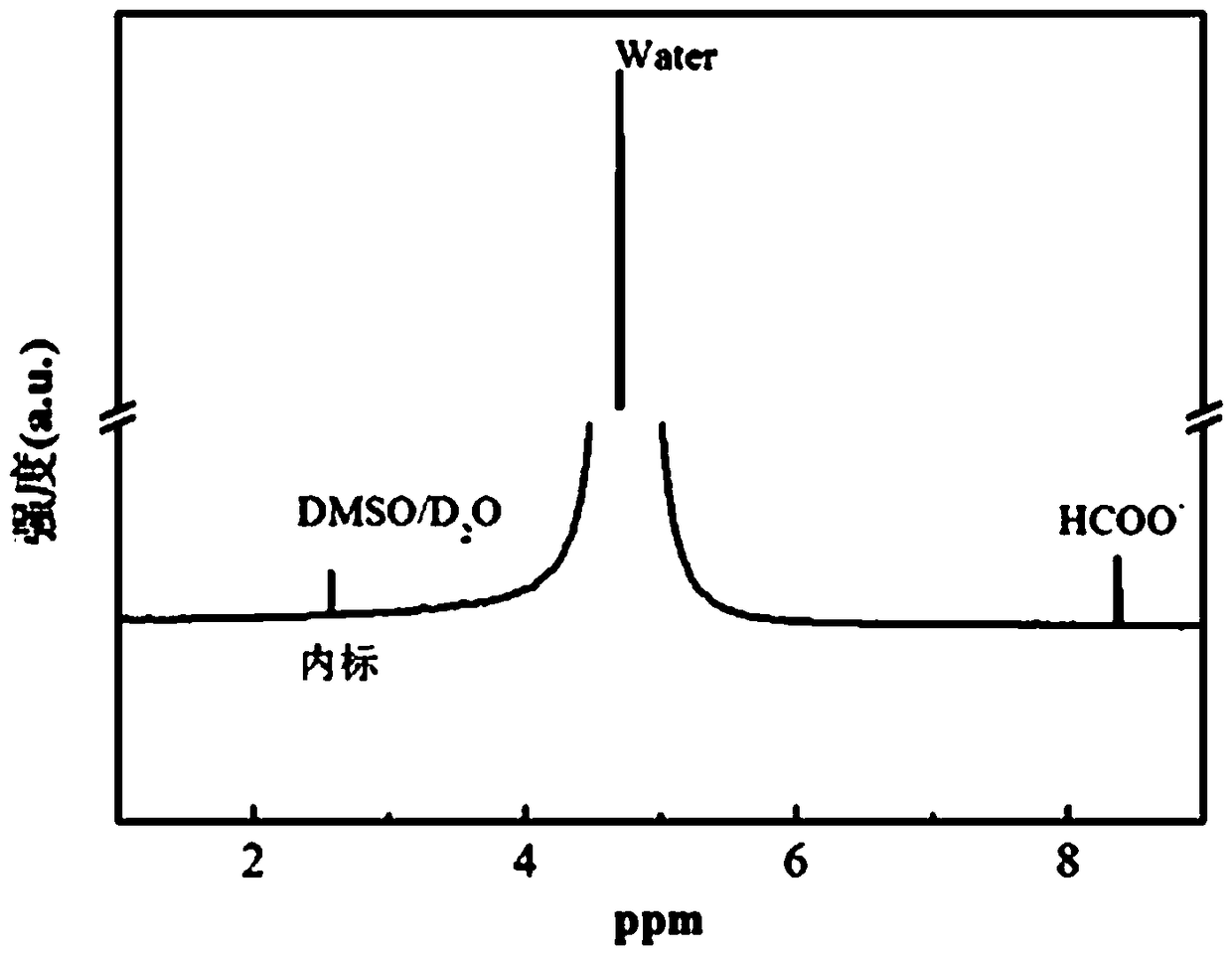
Image
Examples
Embodiment 1
[0027] 10.518 g SnCl 4 ·5H 2 O was dissolved in 60mL distilled water, stirred for 30min until the solution was uniform, and 4 ·5H 2 O molar ratio is 10:1:1 Weigh 0.4034 g CuCl respectively 2 , 0.2284 g thiourea was added to the above solution, magnetically stirred for 3h, and ultrasonically 0.5h. Then the mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel autoclave, heated in water at 180°C for 5 hours, cooled to room temperature after the water heat was completed, soaked in 20 mL of ethanol and water for 1 hour, ultrasonicated for 30 minutes, and then centrifuged. Repeat 5 times until no Cl - Until it is detected, a white precipitate will be obtained, which is dried in a drying oven at 80°C and then ground. The obtained white powder was calcined in a muffle furnace at 500°C for 4h, cooled to room temperature and then ground to obtain modified Cu-S co-doped SnO 2 catalyst. The conductive glass (FTO) was cut to a size of 1 cm×2 cm, and pret...
Embodiment 2
[0029] 10.518 g SnCl 4 ·5H 2 O was dissolved in 60mL distilled water, stirred for 30min until the solution was uniform, and 4 ·5H 2 O molar ratio is 10:2:0.5 and weighs 0.8000 g AlCl respectively 3 and 0.1142 g of thiourea were added to the above solution, magnetically stirred for 5 h, and ultrasonicated for 1 h. Then transfer the mixed solution to a polytetrafluoroethylene-lined stainless steel autoclave, heat it in water at 160 °C for 7 h, cool to room temperature after the end of the water heat, soak in 50 mL of ethanol and water for 1 h, ultrasonic for 30 min, and then centrifuge. Repeat this step 3 times until there is no Cl - Until it is detected, a white precipitate will be obtained, which is dried in a drying oven at 100°C and then ground. The obtained white powder was calcined in a muffle furnace at 400°C for 4h, cooled to room temperature and then ground to obtain modified Al-S co-doped SnO 2 catalyst. The conductive glass (FTO) was cut into a size of 2 cm×2 c...
Embodiment 3
[0031] 10.518 g SnCl 4 ·5H 2 O was dissolved in 60mL distilled water, stirred for 30min until the solution was uniform, and 4 ·5H 2 O molar ratio is 20:1:1 and weighs 0.3050 g MgCl respectively 2 ·6H 2 O and 0.1142 g thiourea were added to the above solution, magnetically stirred for 5 h, and ultrasonicated for 1 h. Then the mixed solution was transferred to a polytetrafluoroethylene-lined stainless steel autoclave, heated in water at 160°C for 3 h, cooled to room temperature after the hydroheat was completed, soaked in 30 mL of ethanol and water for 1 h, and ultrasonicated for 30 min, and then Centrifuge, this step is repeated 5 times until there is no Cl - Until it is detected, a white precipitate will be obtained, which is dried in a drying oven at 100°C and then ground. The obtained white powder was calcined in a muffle furnace at 500°C for 3h, cooled to room temperature and then ground to obtain the modified Mg-S co-doped SnO 2 catalyst. The conductive glass (FTO)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com