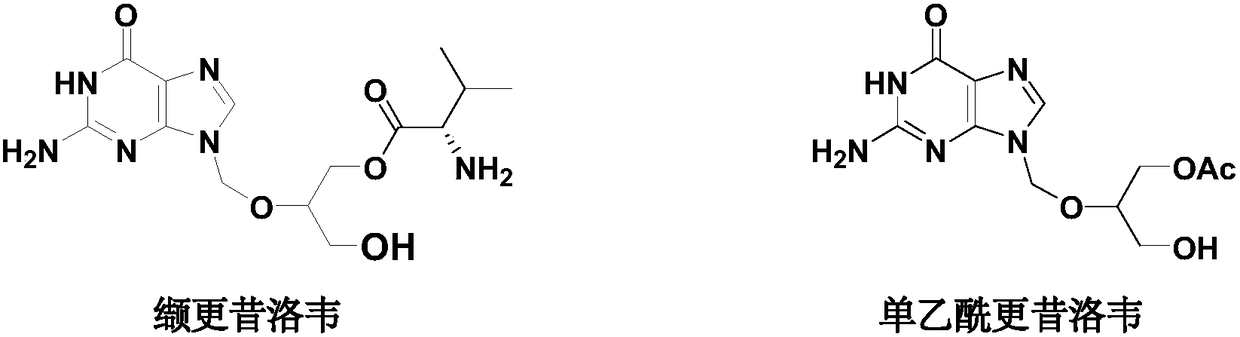
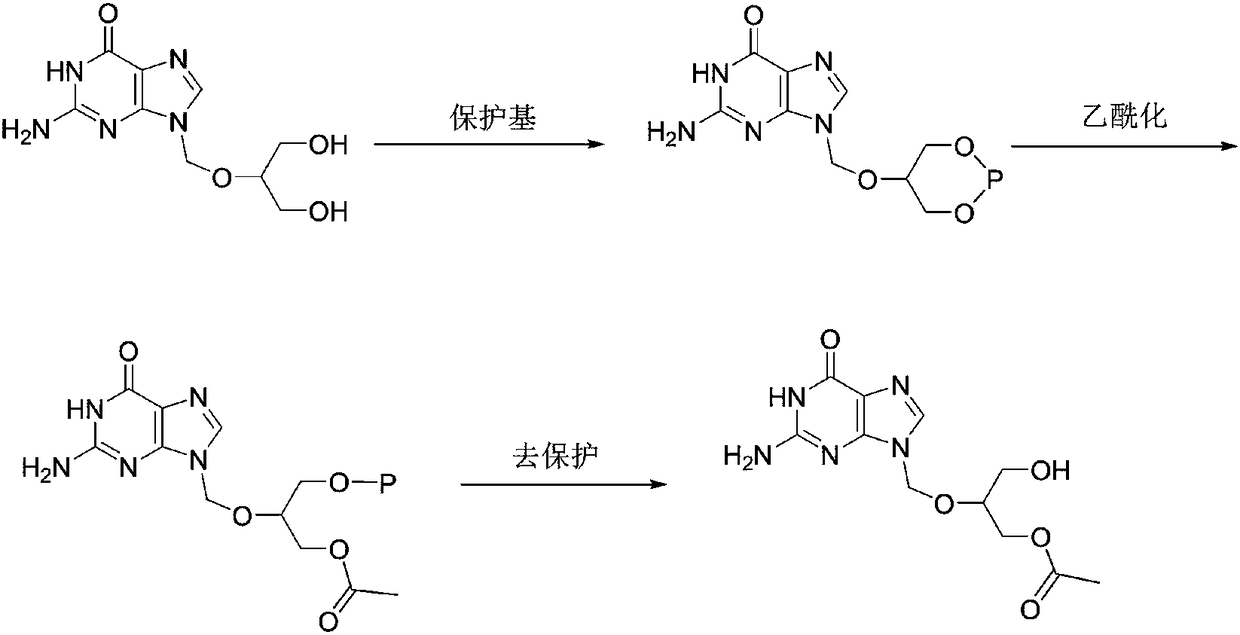
Synthesis method of high-purity monoacetyl ganciclovir
A synthetic method, the technology of ganciclovir, is applied in the field of synthesis of high-purity monoacetyl ganciclovir, which can solve the problem that the purity of monoacetyl ganciclovir is difficult to achieve the ideal quality, affects the quality of valganciclovir, and separates Difficulty in purification and other problems, to achieve the effect of reducing cost and environmental pollution, improving conversion rate and yield, and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
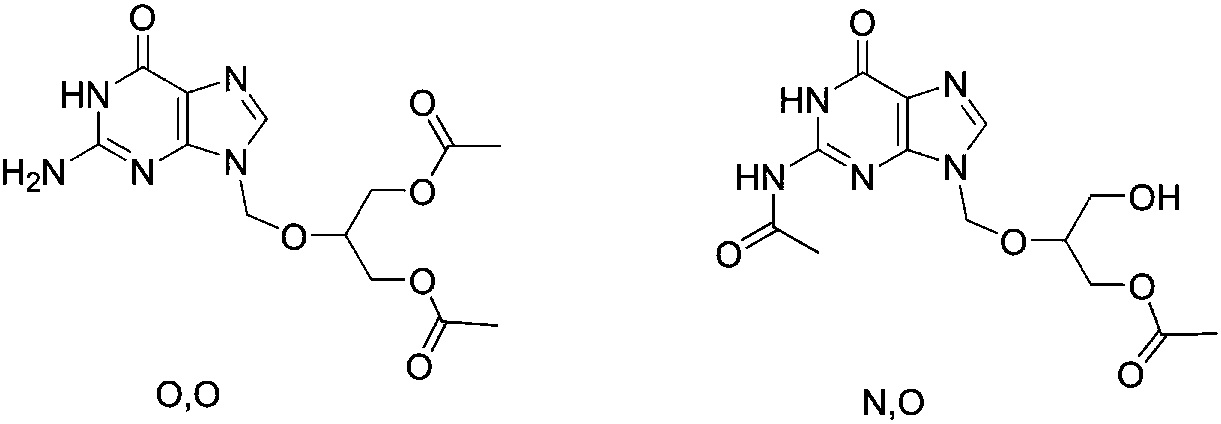
[0029] Add 500.0g (1.96mol, 1.0eq) of ganciclovir, 244.0g (0.98mol, 0.5eq) of dibutyltin oxide, and 5.0kg of tetrahydrofuran into the reaction kettle, heat to reflux for 3 hours, and control the reaction of raw materials in TLC. Completely, lowered to room temperature, added iodine 49.7g (0.196mol, 0.1eq), then added dropwise 337.5g (3.92mol, 2.0eq) of vinyl acetate, stirred at room temperature for 5 hours, controlled monoacetylganciclovir and impurities in HPLC The ratio is 95 / 3, lower the temperature to 0-10°C, add 2.0kg of methanol dropwise, stir at room temperature for 2 hours, concentrate the solvent under reduced pressure, add 3.0kg of ethyl acetate, filter out insoluble matter, and wash the filtrate once with 500g of water. The layers were separated, the aqueous layer was extracted with 500 g of ethyl acetate, the organic layers were combined, concentrated under reduced pressure, and recrystallized from ethanol to obtain 434.5 g of colorless crystals, yield 74.6%, purity...
Embodiment 2
[0034] Add 500.0g (1.96mol, 1.0eq) of ganciclovir, 244.4g (2.35mol, 1.2eq) of trimethyl borate, and 7.5kg of toluene into the reaction kettle, heat to reflux for 5 hours, and the reaction of the raw materials in TLC is complete. , lowered to room temperature, added 396.7g (3.93mol, 2.0eq) of triethylamine, then added 323.7g (2.94mol, 1.5eq) of 1-acetylimidazole, stirred at room temperature for 6 hours, and controlled monoacetylganciclovir in HPLC The ratio of N, O-diacetylganciclovir to N,O-diacetylganciclovir is 94 / 4, lower the temperature to 0-10°C, add 2.0kg of methanol dropwise, after dropping, stir at room temperature for 2 hours, concentrate the solvent under reduced pressure, add 3.0kg of ethyl acetate , washed once with 500g water, separated, the aqueous layer was extracted with 500g ethyl acetate, the organic layers were combined, concentrated under reduced pressure, recrystallized from ethanol to obtain 440.9g of colorless crystals, yield 75.7%, purity 99.2%, mp 133.9...
Embodiment 3
[0036]Add 500.0g (1.96mol, 1.0eq) of ganciclovir, 314.7g (2.15mol, 1.1eq) of triethyl borate, and 5.0kg of 2-methyltetrahydrofuran into the reaction kettle, and heat to reflux for 3 hours. Control the raw materials to react completely, lower to room temperature, add 595.0g (5.88mol, 3.0eq) of triethylamine, then add 421.8g (4.9mol, 2.5eq) of vinyl acetate, stir at room temperature for 6 hours, control monoacetylganciclo in HPLC The ratio of Wei and N,O-diacetylganciclovir is 93 / 5, lower the temperature at 0-10°C, add 2.0kg of ethanol dropwise, after dropping, stir at room temperature for 3 hours, concentrate the solvent under reduced pressure, add 3.0kg of ethyl acetate Esters, washed once with 500g water, separated, the aqueous layer was extracted with 500g ethyl acetate, the organic layers were combined, concentrated under reduced pressure, recrystallized from ethanol to obtain 438.0g of colorless crystals, yield 75.2%, purity 99.1%, mp 133.6-134.5 ℃, MS (m / z) 298.2 (M+H) +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com