Phosphorus-containing cardanol polyglycidyl ether and preparation method thereof
A technology of polyglycidyl ether and glycidyl ether, which is applied in the field of preparation of phosphorus-containing polyglycidyl ether, can solve the problems of less cardanol, migration of cardanol, complicated preparation process, etc., and achieve low cost, good toughness, and improved toughness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Substitution reaction
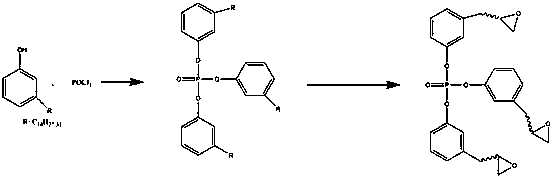
[0025] Add 305g of cardanol into the reaction kettle, then add 300g of dichloromethane solvent, add 5g of triethylamine catalyst, then lower the temperature of the system to about 0°C, add 450g of phosphorus oxychloride dropwise to keep the reaction temperature of the system low After reacting at 10° C. for 3 hours, wash with water, extract, and remove the solvent to prepare tri-cassus-based phosphate.
[0026] (2) Oxidation reaction
[0027] Add 500g of the substance prepared in (1) into the reaction kettle, then add 400g of chloroform, then add 10g of m-chloroperoxybenzoic acid oxidant, the reaction temperature is between 40°C and the reaction time is 5 hours. After the reaction is completed, remove After removing the solvent, washing with water and drying, the phosphorus-containing cardanol polyglycidyl ether is prepared.
[0028] The properties prepared are shown in Table 1
Embodiment 2
[0030] (1) Substitution reaction
[0031] Add 305g of cardanol to the reaction kettle, then add 300g of dichloromethane solvent, add 10g of triethylamine catalyst, then lower the temperature of the system to about 0°C, add 600g of phosphorus oxychloride dropwise to keep the reaction temperature of the system low After reacting at 10° C. for 4 hours, wash with water, extract, and remove the solvent to prepare tri-cassinolate-based phosphate.
[0032] (2) Oxidation reaction
[0033] Add 500g of the substance prepared in (1) into the reaction kettle, then add 400g of chloroform, then add 30g of m-chloroperoxybenzoic acid oxidant, the reaction temperature is between 40°C and the reaction time is 6 hours. After the reaction is completed, remove After removing the solvent, washing with water and drying, the phosphorus-containing cardanol polyglycidyl ether is prepared.
[0034] The properties prepared are shown in Table 1
Embodiment 3
[0036] (1) Substitution reaction
[0037] Add 305g cardanol into the reaction kettle, then add 300g dichloromethane solvent, add 15g triethylamine catalyst, then lower the temperature of the system to about 0°C, add 750g phosphorus oxychloride dropwise to keep the reaction temperature of the system low After reacting at 10° C. for 6 hours, wash with water, extract, and remove the solvent to prepare tricassinolate-based phosphate.
[0038] (2) Oxidation reaction
[0039] Add 500g of the substance prepared in (1) into the reaction kettle, then add 400g of chloroform, then add 45g of m-chloroperoxybenzoic acid oxidant, the reaction temperature is between 40°C and the reaction time is 8 hours. After the reaction is completed, remove After removing the solvent, washing with water and drying, the phosphorus-containing cardanol polyglycidyl ether is prepared.
[0040] The properties prepared are shown in Table 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com