Method for preparing high-purity fenofibric acid in phase transfer catalysis method
A technology of phase transfer catalysis and fenofibric acid, which is applied in the field of catalytic synthesis of fenofibric acid, can solve the problems of large amount of acetone, acid and alkali, and high impurities, so as to reduce use and consumption, reduce solvent loss, and avoid The effect of rectification recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
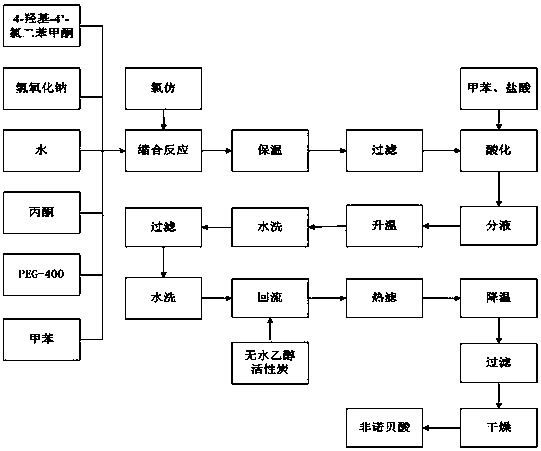
[0029] Add 60 g of water I and 40 g of sodium hydroxide to a four-necked flask equipped with electric stirring, a thermometer, a reflux condenser, and a constant pressure dropping funnel at a speed of 400 r / min, cool down to 40°C, add 50 g of toluene I, 55 g of acetone, 4 -Hydroxy-4'-chlorobenzophenone 40g, PEG-400, 4g; add chloroform 28g dropwise at 47±1°C, keep warm at 47±1°C for 0.5 h after the dropwise addition, and then heat at 59-60°C Incubate for 2 h; add 40 g of water II to the reaction solution, filter, add 55 g of toluene II to the filtrate, acidify with hydrochloric acid (53 g) to pH = 2, then add 55 g of water III to promote the complete dissolution of salts, and let stand to separate the lower water layer , heating the upper layer material liquid to 66±1°C, adding 60g of water IV to cool down to 33°C, standing to separate the lower layer water layer, filtering the upper layer material liquid, washing the filter cake with water until the filtrate pH=6, and obtaining...
Embodiment 2
[0031] Add 55g of water I and 45g of sodium hydroxide into a four-neck flask equipped with electric stirring, thermometer, reflux condenser, and constant pressure dropping funnel at a speed of 450r / min, cool down to 40°C, add 55g of toluene I, 55g of acetone, 4 -Hydroxy-4'-chlorobenzophenone 40g, PEG-400, 3g; add chloroform 32g dropwise at 48±1°C, keep warm at 48±1°C for 0.5 h after the dropwise addition, and then heat at 58-59°C Incubate for 2.5 h; add 50 g of water II to the reaction solution, filter, add 50 g of toluene II to the filtrate, acidify with hydrochloric acid (51 g) to pH = 3, then add 60 g of water III to promote the complete dissolution of salts, and let stand to separate the lower water layer , heating the upper layer material liquid to 68±1°C, adding 50g of water IV to cool down to 35°C, standing to separate the lower layer water layer, filtering the upper layer material liquid, washing the filter cake with water until the filtrate pH=7, and obtaining the wet ...
Embodiment 3
[0033] Add 58g of water I and 42g of sodium hydroxide into a four-necked flask equipped with electric stirring, thermometer, reflux condenser, and constant pressure dropping funnel at a speed of 450r / min, lower the temperature to 40°C, add 60g of toluene I, 60g of acetone, 4 -Hydroxy-4'-chlorobenzophenone 40g, PEG-400, 5g; add chloroform 30g dropwise at 49±1°C, keep warm at 49±1°C for 0.5 h after the dropwise addition, and then heat at 58-59°C Incubate for 2.5 h; add 45 g of water II to the reaction solution, filter, add 60 g of toluene II to the filtrate, acidify with hydrochloric acid (54 g) to pH = 2, then add 50 g of water III to promote the complete dissolution of salts, and let stand to separate the lower water layer , heating the upper material liquid to 69±1°C, adding 55g of water IV to cool down to 32°C, standing to separate the lower water layer, filtering the upper layer material liquid, washing the filter cake with water until the filtrate pH=7, and obtaining the we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com