Organic precipitator as well as preparation method and application thereof
An organic precipitant and sediment technology, applied in the field of organic precipitants and their preparation, can solve the problems of poor selectivity, high content of non-rare earth impurities, adverse effects on the quality of production products, etc., and achieve high yield, fast precipitation, and structural stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
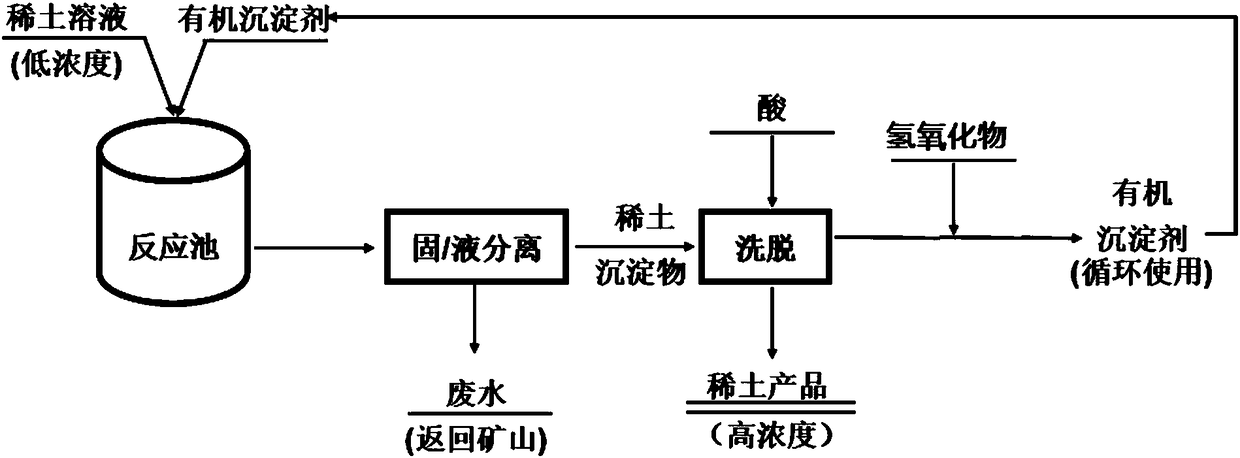
[0042] The present invention also provides a method for preparing the organic precipitating agent described in the above technical scheme, comprising the following steps:
[0043] Alkylphenoxyacetic acid and hydroxide are reacted to obtain an organic precipitant;
[0044] The hydroxide is selected from one or more of lithium hydroxide, sodium hydroxide, potassium hydroxide, calcium hydroxide, magnesium hydroxide and aluminum hydroxide.
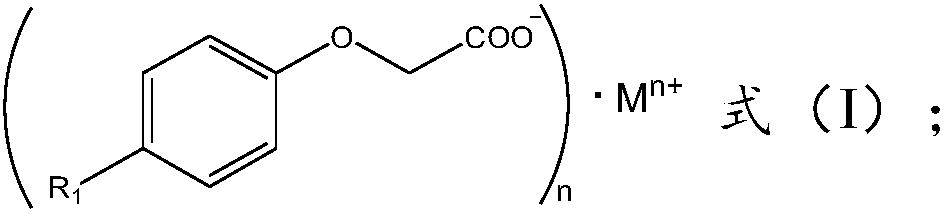
[0045] The alkylphenoxyacetic acid includes one or more of the structural compounds of formula (IV)~formula (VI):
[0046]
[0047] Among them, R 1 , R 2 , R 3 and R' 3 independently selected from substituted straight chain alkyl groups with 1 to 10 carbon atoms, unsubstituted straight chain alkyl groups with 1 to 10 carbon atoms, substituted branched chain alkyl groups with 3 to 10 carbon atoms, An unsubstituted branched alkyl group having 3 to 10 carbon atoms, a substituted aryl group or an unsubstituted aryl group.
[0048] In the ...
Embodiment 1
[0066] 1) Preparation of 1,1,3,3-tetramethylbutylphenoxyacetic acid:
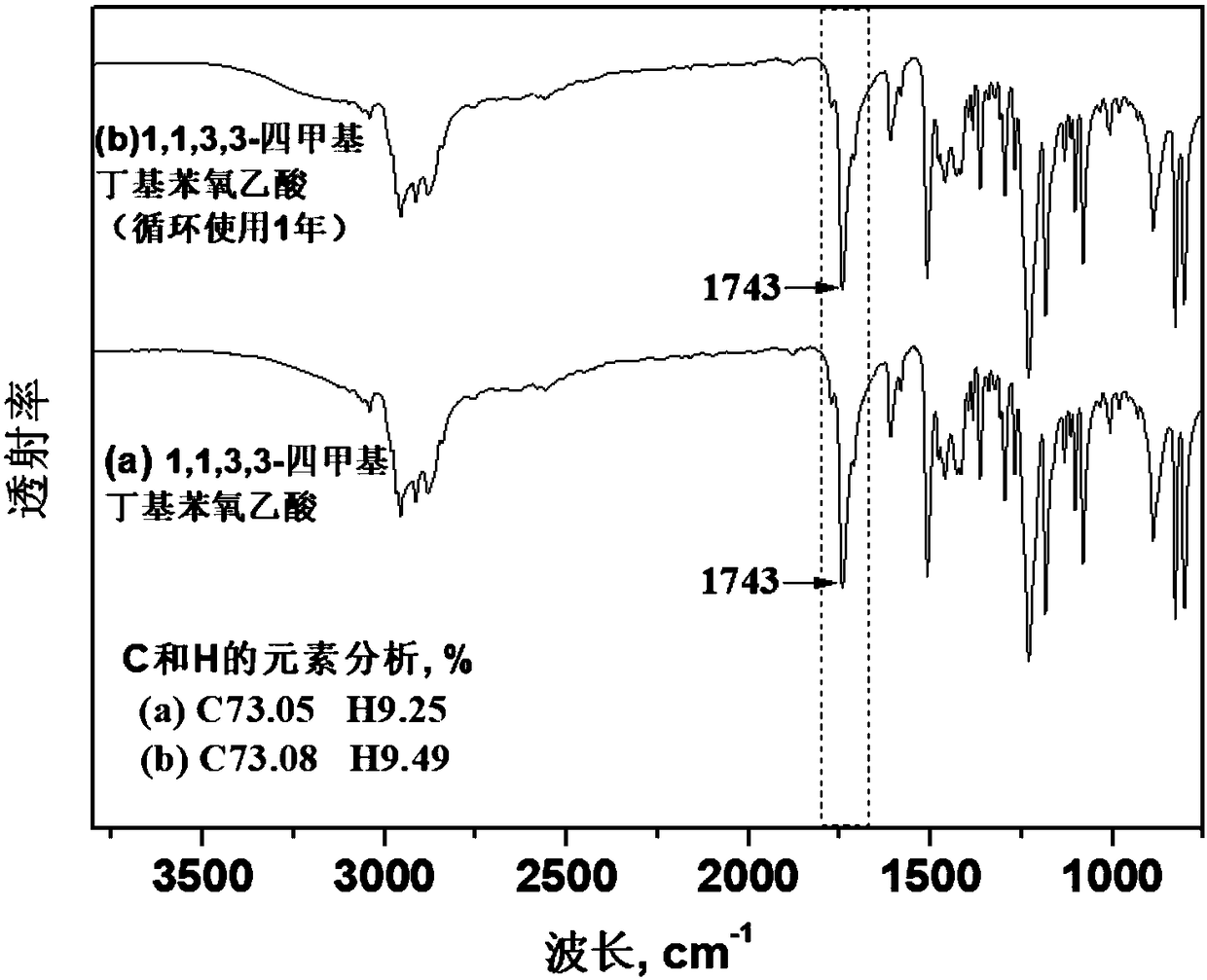
[0067] Add 100 grams of absolute ethanol and 48.4 grams (0.21 mol) of sodium 1,1,3,3-tetramethylbutylphenolate into the reaction vessel. Turn on stirring and heating, raise the temperature to 110° C., and slowly add 37.8 grams (0.32 mol) of sodium chloroacetate into the reaction vessel. After reacting for 1.5 hours, cool to room temperature, distill the solvent of the obtained reaction product under reduced pressure, and then add 50 mL of 6mol / L hydrochloric acid solution for acidification. The acidification temperature is 30°C, and the acidification time is 5 minutes. After three times, distillation under reduced pressure at 160°C gave 1,1,3,3-tetramethylbutylphenoxyacetic acid.
[0068] The 1,1,3,3-tetramethylbutylphenoxyacetic acid prepared in this example was subjected to acid-base titration and NMR detection, the purity was greater than 98%, and it had the structure of formula (IV). In formula (IV), R...
Embodiment 2
[0082] 1) the preparation of p-phenoxydiacetic acid:
[0083] Add 100 grams of absolute ethanol and 22.0 grams (0.20 mol) of hydroquinone into the reaction vessel. Turn on stirring and heating, raise the temperature to 110° C., and slowly add 58.3 grams (0.5 mol) of sodium chloroacetate into the reaction vessel. After reacting for 1.5 hours, cool to room temperature, distill the solvent of the obtained reaction product under reduced pressure, and then add 60 mL of 6mol / L hydrochloric acid solution for acidification. The acidification temperature is 30 ° C, and the acidification time is 5 minutes. After three times, distill under reduced pressure at 160°C to obtain p-phenoxydiacetic acid.
[0084] The p-phenoxydiacetic acid prepared in this example is subjected to acid-base titration and NMR detection, the purity is greater than 98.5%, and it has the structure of formula (V). In formula (V), R 2 Take hydrogen.
[0085] 2) preparation of p-ammonium phenoxydiacetate:
[0086]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com