Preparation and application method of helical chiral all-cis poly 3,5-dimethylol-4-substituted phenylacetylene derivatives without chiral atoms
A technology of helical chirality and dimethylol, which is applied in the field of preparation of helical polymers, can solve the problems of little research and achieve good chiral resolution performance, simple operation and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
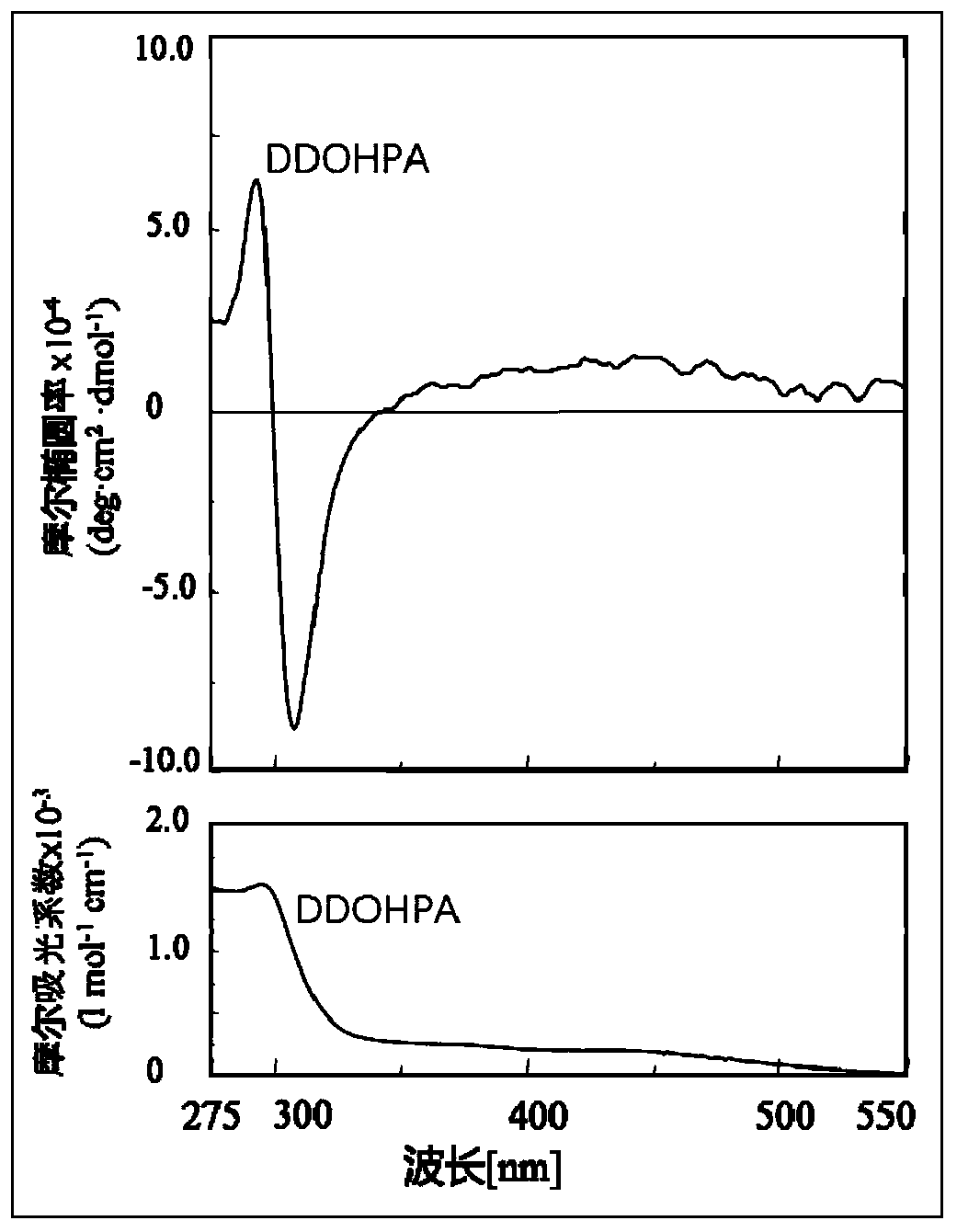
[0029] (1) Preparation of helical chiral all-cis polyacetylene DDOHPA without chiral atom:
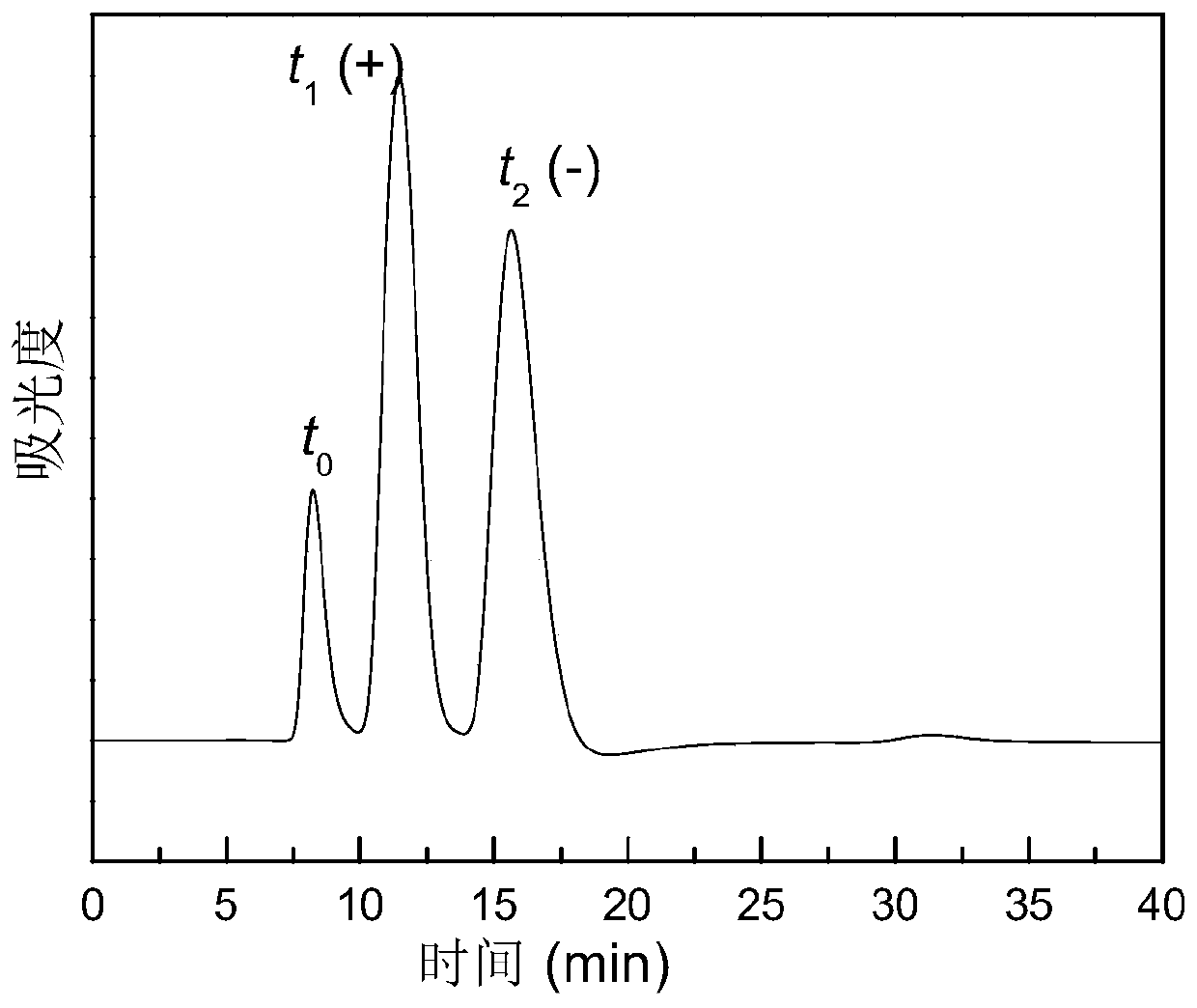
[0030] Measure 50mL (R)-α-methylbenzylamine and bubbling with helium gas for 5 minutes. Weigh 0.50 g of non-optically active all-cis polyphenylene acetylene DDOHPA, and dissolve it in 50 ml (R)-α-methylbenzylamine after bubbling with helium at normal temperature and pressure to make a solution. Pour the solution into a dialysis bag (cellulose dialysis bag, molecular weight cutoff 5000). After the dialysis bag is sealed, the dialysis bag containing the solution is placed in a beaker containing 500 ml of tetrahydrofuran, the beaker is heated to 35 degrees Celsius, and the tetrahydrofuran is replaced every 1 hour. After changing three times, take out the dialysis bag and open it, use a dropper to take out the solution in the dialysis bag and drop it into 500ml methanol to precipitate and precipitate an orange-red precipitate. After centrifugation, the obtained precipitate is vacuum dried fo...
Embodiment 2
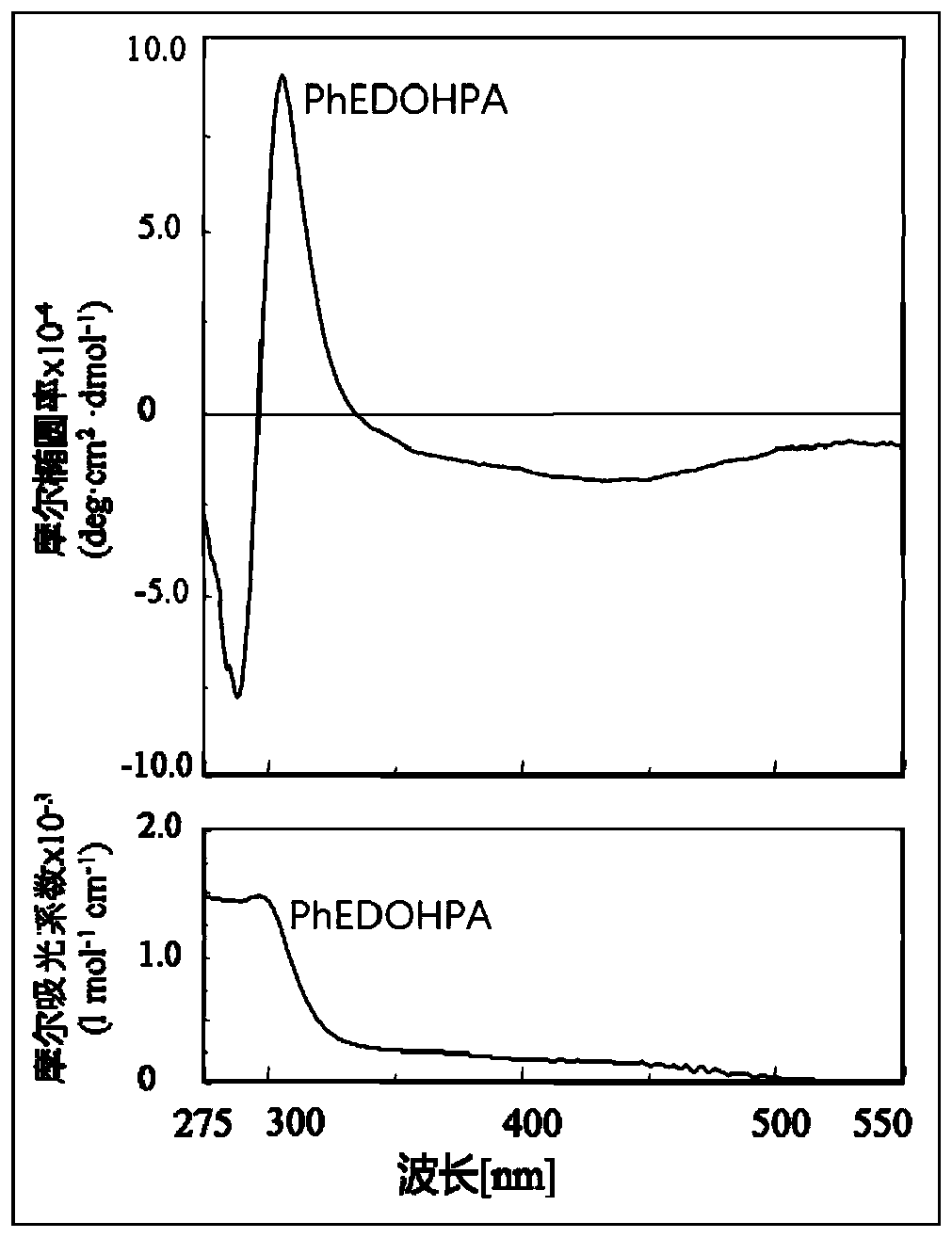
[0035] (1) Preparation method of helical chiral all-cis polyphenylene acetylene PhEDOHPA without chiral atom: Measure 50 mL (S)-α-methylbenzyl alcohol and bubbling with helium for 5 minutes. Weigh 0.50 g of non-optically active all-cis polyphenylene acetylene PhEDOHPA, and dissolve it in 50 ml of (S)-α-methylbenzyl alcohol after bubbling with helium at normal temperature and pressure to make a solution. Pour the solution into a dialysis bag (cellulose dialysis bag, molecular weight cutoff 5000). After the dialysis bag is sealed, the dialysis bag containing the solution is placed in a beaker containing 300 ml of toluene, the beaker is heated to 45 degrees Celsius, and the toluene is replaced every 45 minutes. After changing three times, take out the dialysis bag and open it, use a dropper to take out the solution in the dialysis bag and drop it into 500ml methanol to precipitate and precipitate an orange-red precipitate. After centrifugation, the obtained precipitate is vacuum d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com