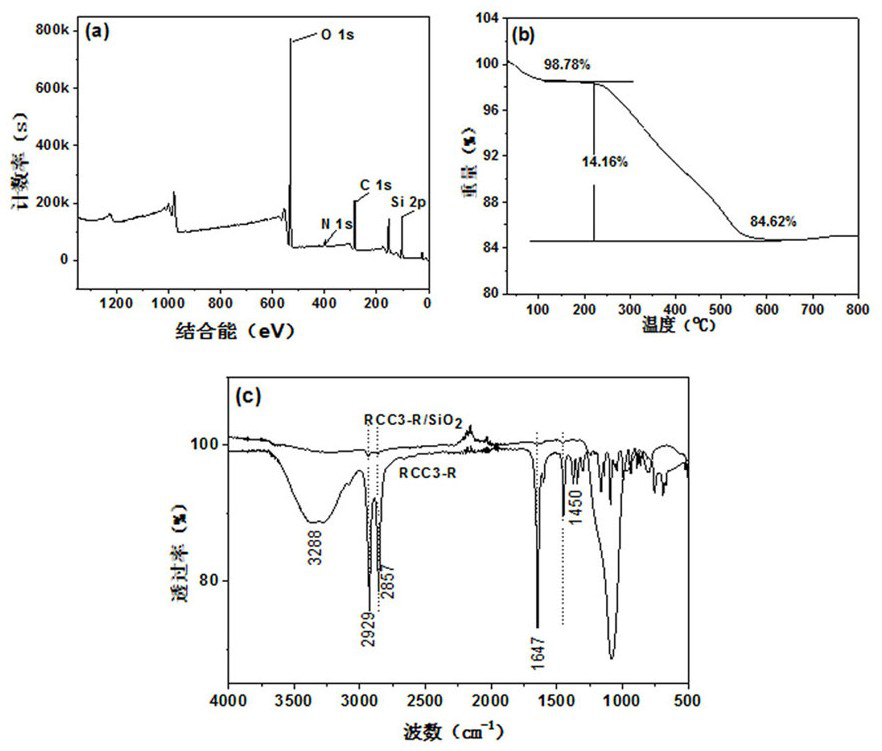
Preparation method and application of multi-mode and multifunctional molecular cage high performance liquid chromatography stationary phase
A technology of high performance liquid chromatography and molecular cage, which is applied in the field of molecular cage high performance liquid chromatography stationary phase to achieve the effect of good chiral separation performance and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
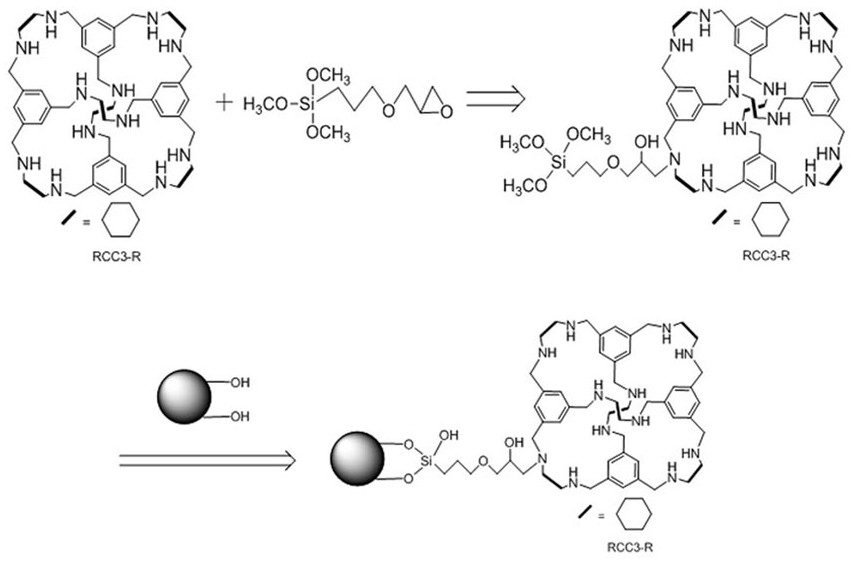
[0025] Preparation of molecular cage high performance liquid chromatography stationary phase with a loading capacity of 5%-30%, see the preparation process figure 1 ;
[0026] (1) Preparation of molecular cage HPLC stationary phase with a loading capacity of 5%: dissolve 0.05 g RCC3-R reduced cyclohexanediamine molecular cage in 20 mL toluene, and then add 0.3 g epoxyalkyltrimethyl Silane, react at 60°C for 24 h, continue to add 1 g of hydrochloric acid-activated silica gel, react at 110°C for 24 h, filter through a sand core funnel, wash with toluene and absolute ethanol, and dry at 60°C to prepare a molecular cage with a loading capacity of 5%. RCC3-R bonded silica gel stationary phase;
[0027] (2) Prepare a molecular cage HPLC stationary phase with a loading capacity of 15%: dissolve 0.3 g RCC3-R reduced cyclohexanediamine molecular cage in 50 mL toluene, and then add 0.3 g epoxyalkyltrimethyl Silane, react at 60°C for 24 h, continue to add 1.7 g of hydrochloric acid-act...
Embodiment 2
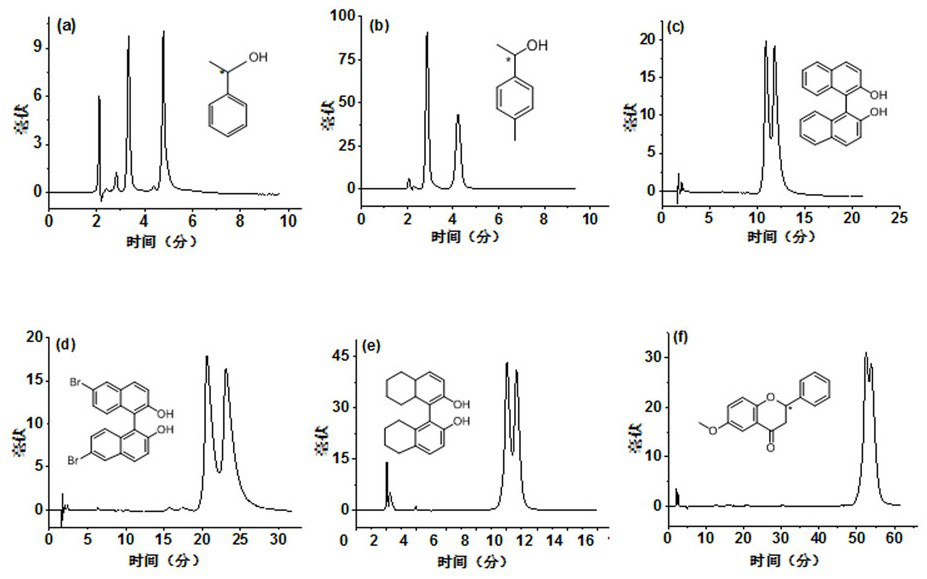
[0031] Using the molecular cage RCC3-R bonded silica gel stationary phase with a loading capacity of 15% obtained in Example 1 to test the resolution effect of chiral compounds, the chromatogram of the resolution is shown in image 3 , 4; image 3 It is the stationary phase of the present invention under normal phase chromatographic conditions for chiral compounds (a) 1-phenylethanol, (b) p-methyl-1-phenylethanol, (c) 1,1'-2-naphthol, ( d) Chromatographic separation of dibromo-binaphthol, (e) 8H-1,1′-binaphthol, and (f) 6-methoxyflavanone. Mobile phase: (a, b) ethanol / n-hexane (5 / 95, v / v); (c, d) ethanol / n-hexane (20 / 80, v / v); (e) ethanol / n-hexane (5 / 95 , v / v); (f) ethanol / n-hexane (2 / 98, v / v); flow rate: 1.0 mL / min; detection wavelength: 254 nm; temperature: 25 ℃; image 3 It can be proved that the complex interaction between the RCC3-R bonded silica chiral stationary phase and the chiral compounds of alcohols, naphthols and flavanones makes the stationary phase suitable f...
Embodiment 3
[0034] Using the molecular cage RCC3-R bonded silica gel stationary phase with a loading capacity of 15% obtained in Example 1, the separation effect of p-alkylbenzene compounds in the normal phase and reverse phase chromatography separation modes, the separation effect is shown in Figure 5 ; Figure 5 The chromatographic conditions and separated compounds are: mobile phase: methanol / water (40:60, v / v); flow rate: 1.0 mL / min; detection wavelength: 254 nm; analyte: 1. toluene; 2. ethylbenzene ; 3. Propylbenzene; 4. Butylbenzene; 5. Pentylbenzene.
[0035] It can be seen from Example 3 that the good separation of five benzenecycloalkyl homologues shows that the RCC3-R bonded silica gel stationary phase has better reversed-phase chromatographic separation mode performance and methylene selectivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com