Continuous production method for isobornyl methacrylate
A technology of isobornyl methacrylate and methacrylic acid, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of high prices of borneol and isobornyl, reduced value of atomic utilization, and environmental pollution Large and other problems, to achieve the effect of high utilization rate of raw materials, reduction of side reactions, and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
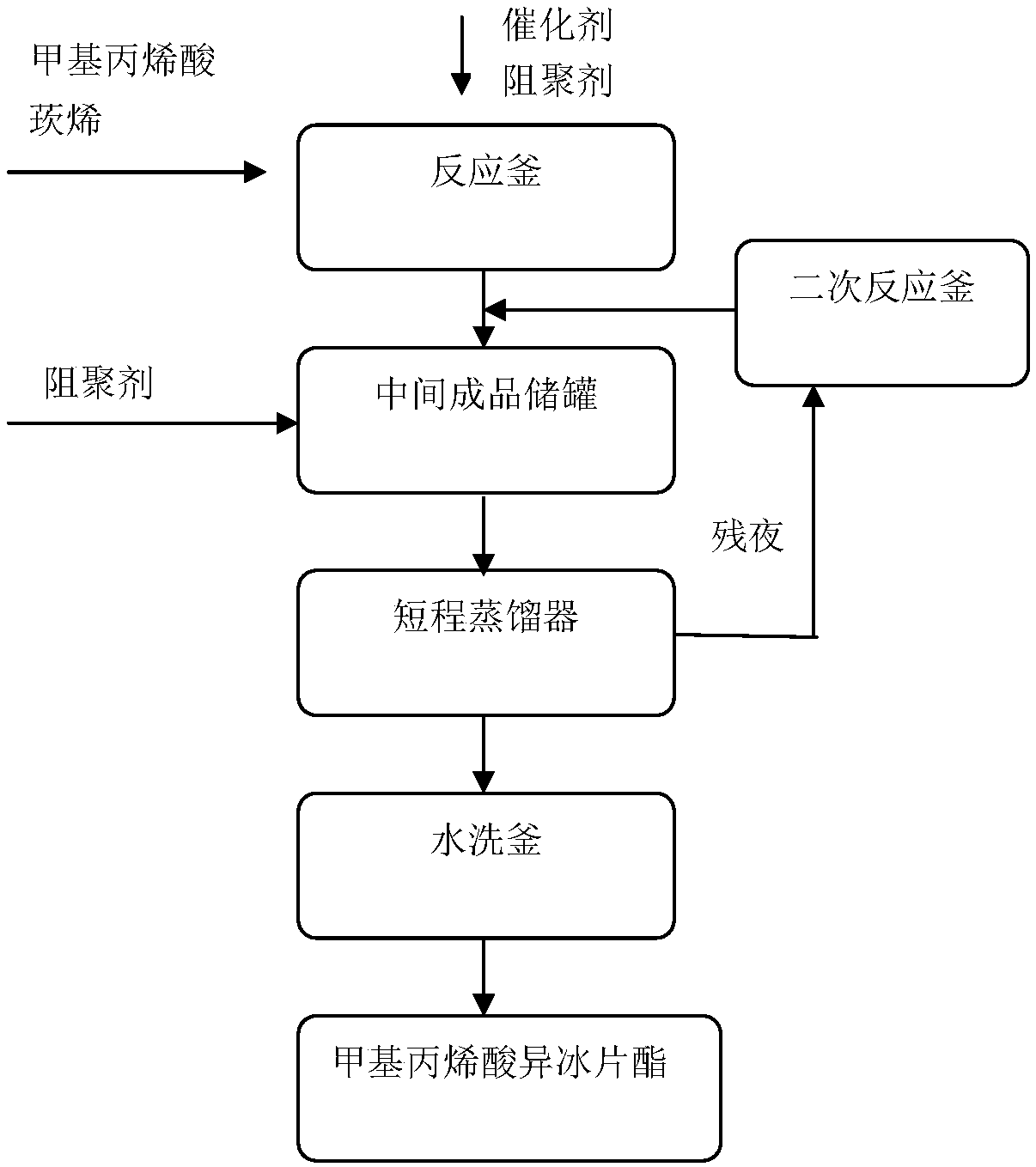
[0021] Such as figure 1 Shown, a kind of continuous production method of isobornyl methacrylate comprises the following steps: (1) methacrylic acid and refined camphene are dropped into reactor, dissolve at room temperature, and add a certain amount of polymerization inhibitor after completely dissolving Agent and catalyst, heated to 60 degrees Celsius to react, no heat is released during the reaction, after 3 hours of reaction, stop the reaction, filter and recover the catalyst to obtain the reaction mixture, temporarily store the reaction mixture in the semi-finished temporary storage tank;
[0022] (2) In the treated reaction mixture, add a polymerization inhibitor, then pump it into a short-path still and steam excess methacrylic acid and unreacted camphene under a negative pressure of 0.01MPa. Collect the distillate at 113-120°C under 0.6kPa, then wash the distillate through water to remove the acid liquid in it, and control the acidity of the product at 0.05, which is is...
Embodiment 2
[0027] Produce isobornyl methacrylate with the method of embodiment 1, difference is only: methacrylic acid and amphene react 4h at 50 ℃, the mass ratio of methacrylic acid and amphene is 5:11, catalyst ferric chloride The addition amount of the polymerization inhibitor ZJ-705 is 0.2wt% of the total weight of methacrylic acid and amphene, and the addition amount of the polymerization inhibitor ZJ-705 is 0.3wt% of the total weight of methacrylic acid and amphene.
Embodiment 3
[0029] Isobornyl methacrylate is produced by the method of Example 1, the difference is only: methacrylic acid and amphene are reacted at 70°C for 2h, the mass ratio of methacrylic acid and amphene is 9:11, and the catalyst ferric chloride The addition amount of the polymerization inhibitor ZJ-705 is 0.4wt% of the total weight of methacrylic acid and amphene, and the addition amount of the polymerization inhibitor ZJ-705 is 0.5wt% of the total weight of methacrylic acid and amphene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com