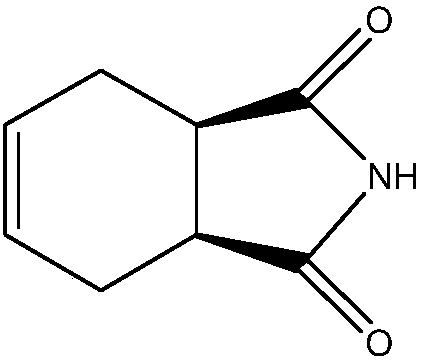
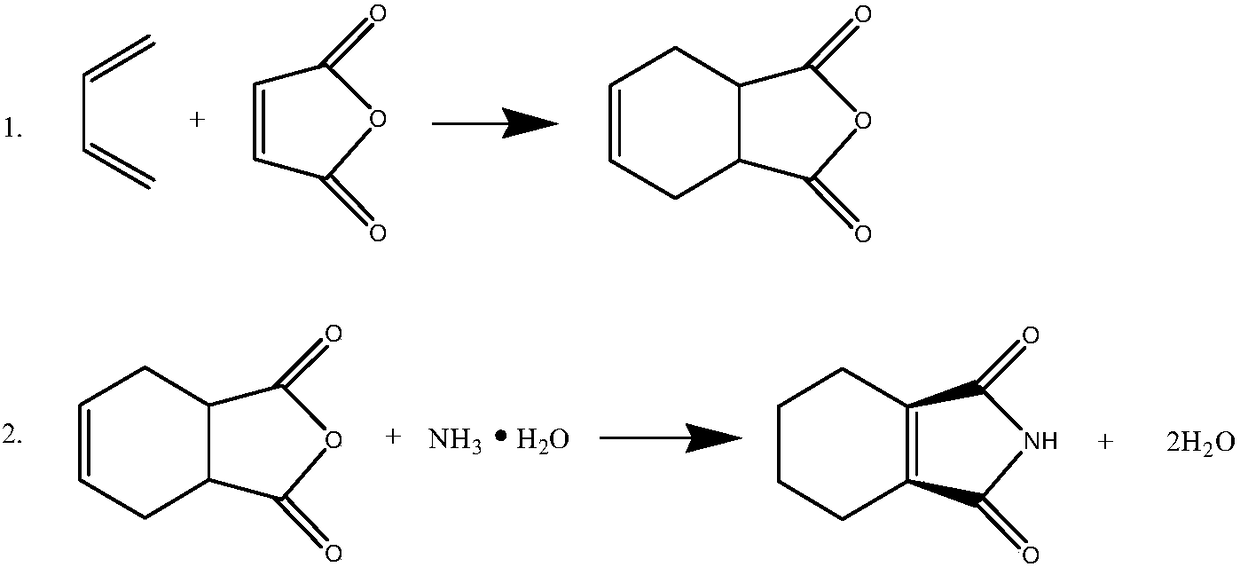
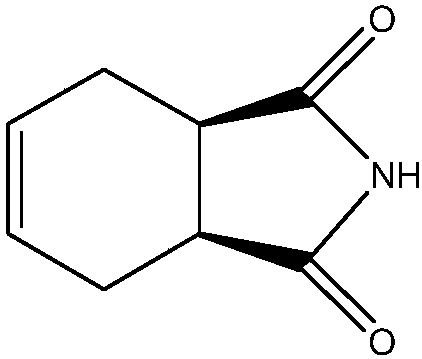
Synthesizing method of cis-1,2,3,6-tetrahydrophthalimide
A technology of tetrahydrophthalimide and phthalimide, which is applied in the direction of organic chemistry, can solve the problems of difficult application in the field of biology and materials science, low product quality, difficult separation, etc., and achieve Avoid polymerization side reaction, high conversion rate, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] Add 30 g (0.20 mol) of phthalimide, 600 ml of N,N-dimethylformamide and 9 g of Raney nickel into a 1 L stainless steel magnetically stirred autoclave, and seal the autoclave. After the air in the kettle was replaced with nitrogen, stirring was started to raise the temperature.
[0035] When the temperature rises to 110°C, the gauge pressure is 0MPa. At this time, hydrogen gas was introduced, and the temperature in the kettle was ensured to be between 105-115°C. After 70 minutes, the pointer of the pressure gauge is raised, the hydrogen inlet valve is closed, and the heat preservation and stirring are continued for 30 minutes, and the pressure gauge returns to zero.
[0036] Re-open the hydrogen inlet valve, and after 2 hours, the pointer of the pressure gauge rises, close the hydrogen inlet valve, continue to keep warm and stir for 30 minutes, and the pressure gauge returns to zero.
[0037] In this way, whenever the pressure gauge has pressure, stop the hydrogen flow...
example 2
[0041] Add 30 g (0.37 mol) of phthalimide, 600 ml of N,N-dimethylformamide and 5 g of Raney nickel into a 1 L stainless steel magnetically stirred autoclave, and seal the reaction kettle. After the air in the kettle was replaced with nitrogen, stirring was started to raise the temperature.
[0042] When the temperature rises to 110°C, the gauge pressure is 0MPa. At this time, hydrogen gas was introduced, and the temperature in the kettle was ensured to be between 105-115°C. After 1 hour, the pointer of the pressure gauge is raised, the hydrogen inlet valve is closed, and the heat preservation and stirring are continued for 40 minutes, and the pressure gauge returns to zero.
[0043] Re-open the hydrogen inlet valve, and after 45 minutes, the pointer of the pressure gauge rises, close the hydrogen inlet valve, continue to keep warm and stir for 30 minutes, and the pressure gauge returns to zero.
[0044] In this way, whenever the pressure gauge has pressure, stop the hydrogen...
example 3
[0048] Add 30 g (0.37 mol) of phthalimide, 600 ml of N,N-dimethylformamide and 5 g of Raney nickel into a 1 L stainless steel magnetically stirred autoclave, and seal the autoclave. After the air in the kettle was replaced with nitrogen, stirring was started to raise the temperature.
[0049] When the temperature rises to 80°C, the gauge pressure is 0MPa. At this time, hydrogen gas was introduced, and the temperature in the kettle was ensured to be between 80-85°C. After 20 minutes, the pointer of the pressure gauge is raised, the hydrogen inlet valve is closed, and the heat preservation and stirring are continued for 20 minutes, and the pressure gauge returns to zero.
[0050] Re-open the hydrogen inlet valve, and after 18 minutes, the pointer of the pressure gauge rises, close the hydrogen inlet valve, continue to keep warm and stir for 20 minutes, and the pressure gauge returns to zero.
[0051] In this way, whenever the pressure gauge has pressure, stop the hydrogen flow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com