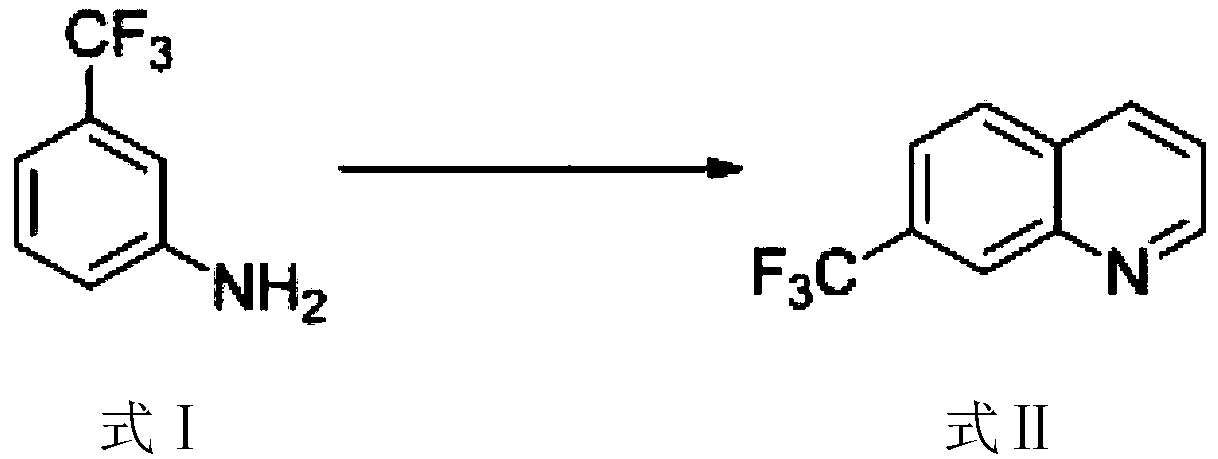5-bromo-7-trifluoromethyl quinoline synthetic method
A technology of trifluoromethylquinoline and synthesis method, applied in the direction of organic chemistry, etc., to achieve the effects of reasonable process selection, convenient operation and post-treatment, and simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
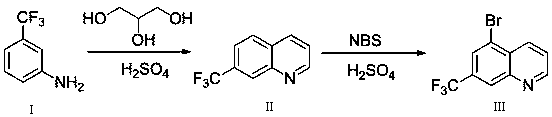
[0024] like figure 1 As shown, the synthetic method of formula VII provided by the present invention: 5-bromo-7-trifluoromethylquinoline comprises the following steps:
[0025] The synthesis of step (1) 7-trifluoromethylquinoline:
[0026] The raw material m-trifluoromethylaniline (formula I) is subjected to Skraup condensation to obtain 7-trifluoromethylquinoline (formula II);
[0027] Synthesis of step (2) 5-bromo-7-trifluoromethylquinoline:
[0028] The 7-trifluoromethylquinoline (formula II) obtained in step (1) is subjected to a bromination reaction to obtain 5-bromo-7-trifluoromethylquinoline (formula III).
[0029] In other words, the synthetic method of 5-bromo-7-trifluoromethylquinoline provided by the present invention mainly comprises the following steps:
[0030] First, use m-trifluoromethylaniline as a raw material to obtain 7-trifluoromethylquinoline through Skraup condensation, and then obtain the obtained 7-trifluoromethylquinoline through bromination reacti...
Embodiment 1
[0040] The first step reaction is a Skraup condensation reaction, the reactants are m-trifluoromethylaniline and glycerol, the reaction solvent is sulfuric acid, the reaction temperature is 135°C, and the reaction time is 4h.
[0041] The second step reaction is bromination reaction, the reagent used is N-bromosuccinimide (NBS), the reaction solvent is concentrated sulfuric acid, the reaction temperature is 70°C, and the reaction time is 4h.
Embodiment 2
[0043] Synthesis of 7-trifluoromethylquinoline
[0044] H 2 SO 4 (13.7g, 0.14mol) was slowly added to glycerin (8.63g, 0.094mol), the temperature should not exceed 70°C, then m-trifluoromethylaniline (5.00g, 0.031mol) was added, the temperature was raised to 85°C, and the reaction was carried out for 40min. Potassium iodide (0.30g, 1.80mmol), iodine (0.34g, 1.34mmol) and water (1.50mL) were added, the temperature was raised to 135°C, and the reaction was carried out for 4h. Cool to room temperature, pour into ice to quench the reaction, and filter through celite. Adjust the pH of the filtrate to 7 with ammonia water, filter with suction, and fully extract the filtrate with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate and concentrated, and the residue was subjected to column chromatography to obtain 7-trifluoromethylquinoline (3.50 g, 69%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com