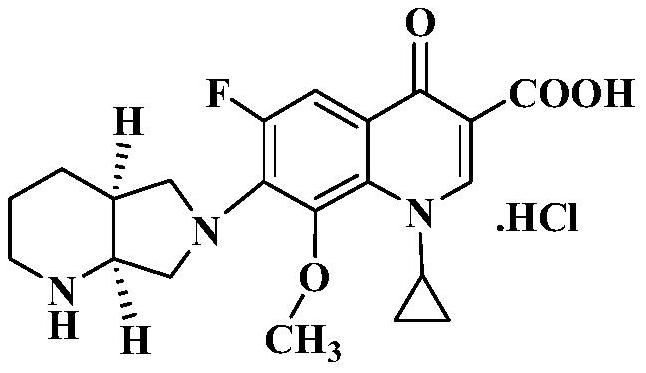
A kind of purification method of moxifloxacin hydrochloride and the preparation method of moxifloxacin hydrochloride
A technique for moxifloxacin hydrochloride and a purification method, which is applied in the field of drug synthesis, can solve the problems of low purity, low yield, and large loss of moxifloxacin hydrochloride, and achieve simple purification methods, high purification efficiency, and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The present embodiment provides a kind of purification method of moxifloxacin hydrochloride, it comprises the following steps:
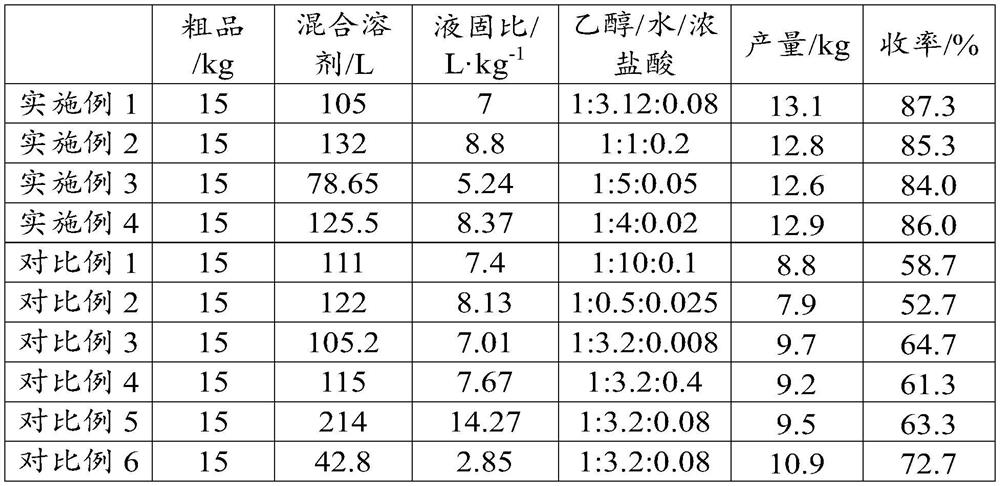
[0032] S1. Add 132L ethanol / water / concentrated hydrochloric acid solution (obtained by mixing 60L ethanol, 60L purified water and 12L concentrated hydrochloric acid) to 15kg crude moxifloxacin hydrochloride, stir and heat up to 90°C to dissolve, and reflux for 30min.
[0033] S2. Filtrate hot, cool the filtrate to 5° C., and stir for 2 hours to carry out crystallization.
[0034] S3. Centrifuge the crystallized solution for 30 min at 2000 rpm to obtain a solid precipitate.
[0035] S4. The above solid precipitate was air-dried at 50° C. for 12 hours to obtain 13.1 kg of light yellow moxifloxacin hydrochloride product with a yield of 87.3%.
Embodiment 2
[0037] The present embodiment provides a kind of purification method of moxifloxacin hydrochloride, it comprises the following steps:
[0038] S1. Add 132L ethanol / water / concentrated hydrochloric acid solution (obtained by mixing 60L ethanol, 60L purified water and 12L concentrated hydrochloric acid) to 15kg of crude moxifloxacin hydrochloride, stir and heat up to 80°C to dissolve, and reflux for 60min.
[0039] S2. Filtrate hot, cool the filtrate to 0°C, and stir for 1 hour to crystallize.
[0040] S3. Centrifuge the crystallized solution at 3000 rpm for 15 min to obtain a solid precipitate.
[0041] S4. The above-mentioned solid precipitate was air-dried at 80° C. for 10 h to obtain 12.8 kg of light yellow moxifloxacin hydrochloride product with a yield of 85.3%.
Embodiment 3
[0043] The present embodiment provides a kind of purification method of moxifloxacin hydrochloride, it comprises the following steps:
[0044] S1. Add 78.65L of ethanol / water / concentrated hydrochloric acid solution (obtained by mixing 13L of ethanol, 65L of purified water and 0.65L of concentrated hydrochloric acid) to 15kg of crude moxifloxacin hydrochloride, stir and heat up to 80°C to dissolve, and reflux for 40min.
[0045] S2. Filtrate hot, cool the filtrate to 10°C, and stir for 2 hours to carry out crystallization.
[0046] S3. Centrifuge the crystallized solution for 30 min at 2000 rpm to obtain a solid precipitate.
[0047] S4. The above solid precipitate was air-dried at 70° C. for 10 h to obtain 12.6 kg of light yellow moxifloxacin hydrochloride product with a yield of 84.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com