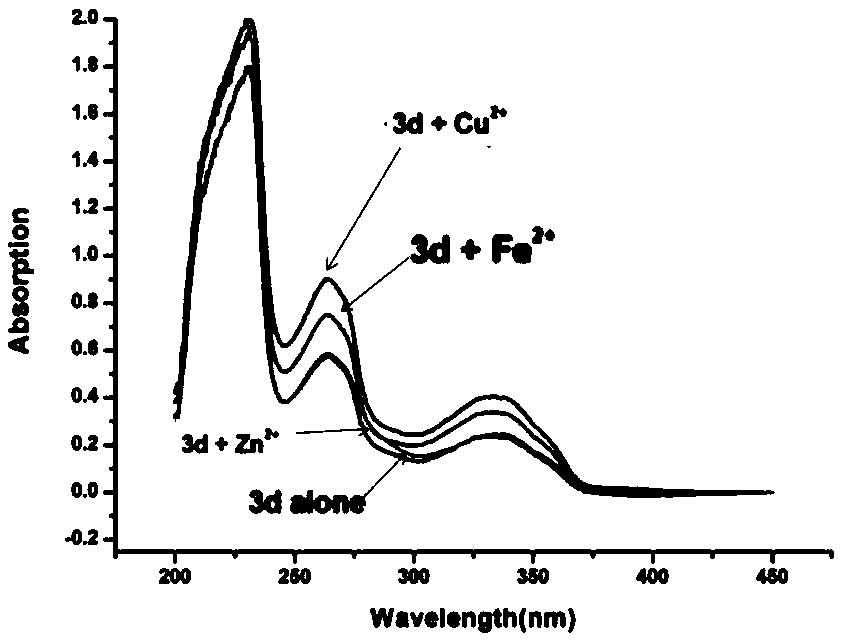
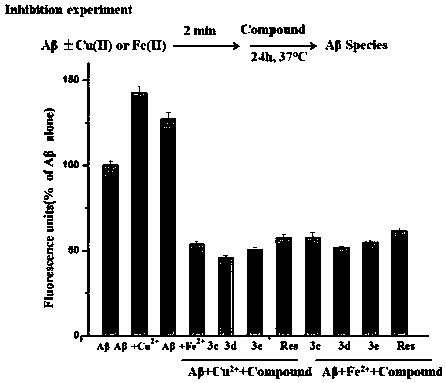
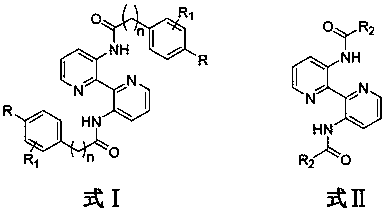
3,3'-disubstituted bipyridine derivatives, and preparation method and application thereof
A bipyridine and derivative technology, which is applied in the field of 3,3'-disubstituted bipyridine derivatives and their preparation, can solve the problems of neuron loss of cognitive function, decline, insufficient explanation for the accumulation of peptides, etc. The method is simple, the raw material is cheap, and the effect of low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of N,N'-[(2,2'-bipyridyl)-3,3'-diyl]bis(4-chlorobenzamide)(compound 3a)
[0030] Add 0.20g of 4-chlorobenzoic acid and 0.14mL of N,N-diisopropylethylamine (DIEA) into a round-bottomed flask containing 8mL of DMF, stir to dissolve the solid completely, and cool to 0-5°C with an ice bath . After adding 0.21 g of 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU), stir for 5 minutes, then add 3,3'-di Amino-2,2'-bipyridine 0.10g, stirred in ice bath for 10min, then naturally warmed to room temperature, followed by TLC, after the reaction was completed, the reaction solution was poured into 20mL of ice water, stirred, and a light yellow solid precipitated. The solid was suction filtered, washed with water and dried. The crude product was purified by silica gel chromatography to obtain 3a as a light yellow powder with a yield of 81%. 1 H NMR (400MHz, CDCl 3 ): δ14.35(s,2H),9.39(s,2H),8.42(s,2H),7.65(d,J=8.0Hz,4H),7.47(d,J=8.0Hz,4H)...
Embodiment 2
[0032] Embodiment 2: the synthesis of compound 3b
[0033] The method was the same as in Example 1, except that benzoic acid was used instead of 4-chlorobenzoic acid, and the crude product was purified by silica gel column chromatography to obtain light yellow solid 3b with a yield of 84%. 1 H NMR (400MHz, CDCl 3)δ14.56(s, 2H), 9.43(d, J=7.9Hz, 2H), 8.42(s, 2H), 8.08(d, J=5.7Hz, 4H), 7.57(d, J=7.0Hz, 6H),7.48-7.45(m,2H)..ESI-MS m / z:395.5[M+H] + .
[0034]
Embodiment 3
[0035] Embodiment 3: the synthesis of compound 3c
[0036] The method was the same as in Example 1, except that 3,4-dimethoxybenzoic acid was used instead of 4-chlorobenzoic acid, and the crude product was purified by silica gel chromatography to obtain 3c as a white solid with a yield of 85%. 1 H NMR (400MHz, CDCl 3 ):δ14.35(s,2H),9.39(s,2H),8.42(s,2H),7.64(s,4H),7.45(s,2H),7.02(s,2H),3.99(s, 12H).ESI-MS m / z:515.5[M+H] + .
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com