Drop, tablet and powder type N-acetylneuraminic acid anti-virus composition
A technology of acetylneuraminic acid and composition, which is applied in the direction of antiviral agents, drug combinations, active ingredients of hydroxyl compounds, etc., and can solve problems such as short residence time, inability to satisfy different user groups, and single dosage form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
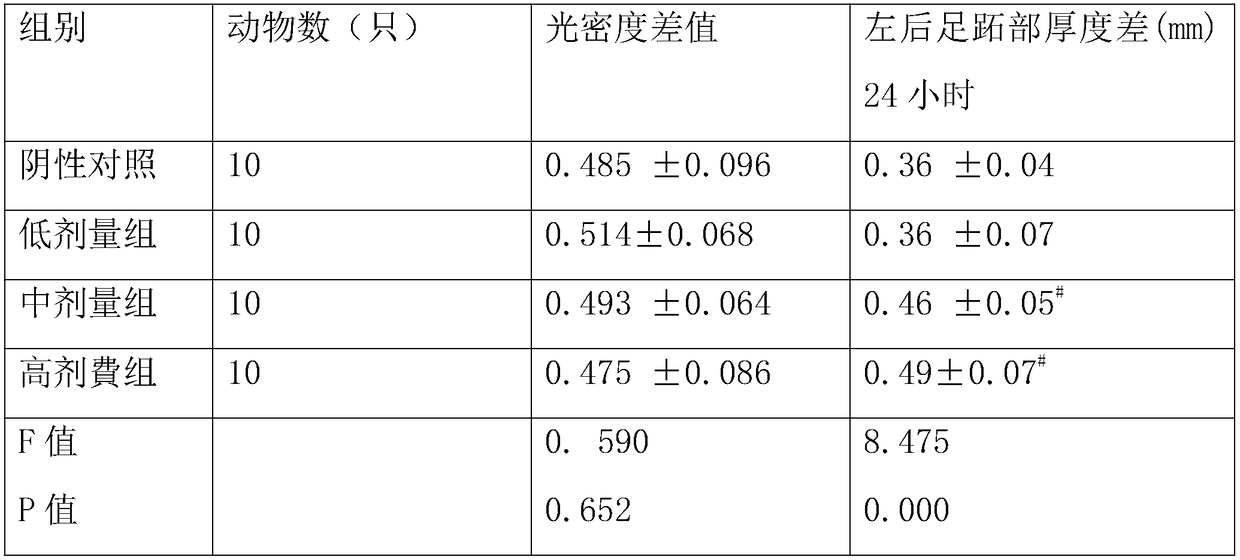
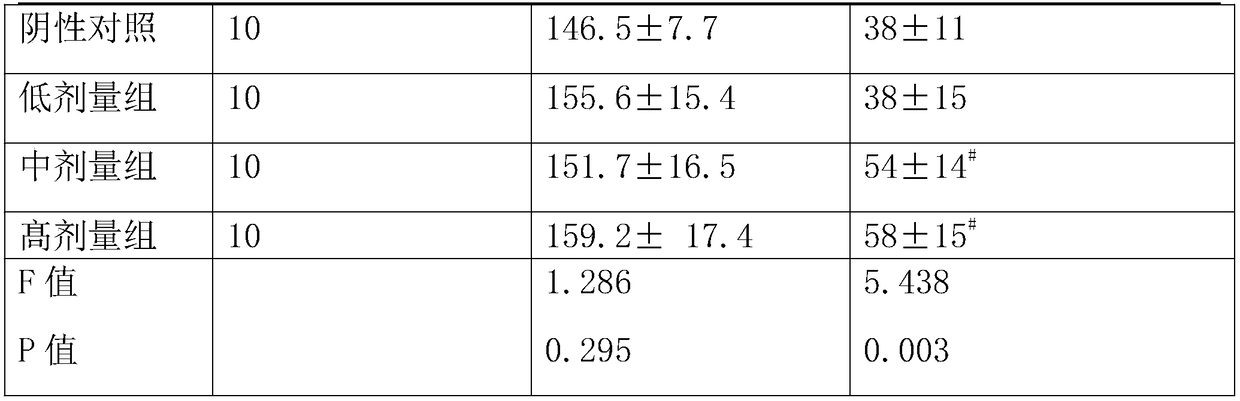
[0039] A drop-type N-acetylneuraminic acid antiviral composition described in this embodiment is characterized in that it includes the following components in weight percentage: 0.6% of lactoferrin, 12% of N-acetylneuraminic acid, immune 3% globulin, 10% glycerin, 13.81% fructooligosaccharide and 40% elderberry juice concentrate, further including 2.5% vitamin C, 0.03% niacin, 5% yam, 0.5‰ potassium sorbate and 5% tangerine peel, It further includes 5% of honeysuckle, 5% of licorice, 0.01% of taurine and 3% of zinc-containing components including zinc gluconate.
Embodiment 2
[0041] A drop-type N-acetylneuraminic acid antiviral composition described in this embodiment is characterized in that it comprises the following components in weight percentage: lactoferrin 8%, N-acetylneuraminic acid 5%, immune Globulin 35%, glycerin 5%, fructooligosaccharide 10.1% and elderberry juice concentrate 10.1%, further including vitamin C 2.5%, niacin 0.18%, yam 5%, potassium sorbate 1‰ and tangerine peel 5%, It further includes 5% of honeysuckle, 5% of licorice, 0.05% of taurine and 8.069% of zinc-containing components including zinc gluconate.
Embodiment 3
[0043] A tablet-type N-acetylneuraminic acid antiviral composition described in this example includes the following components by weight percentage: lactoferrin 0.2%, N-acetylneuraminic acid 1.5%, immunoglobulin 1 % and elderberry powder 5%, further including vitamin C45%, niacin 1.8%, further including honeysuckle extract 5%, licorice extract 5%, further including taurine 0.5%, zinc gluconate 0.5%, further including Xylitol 34.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com