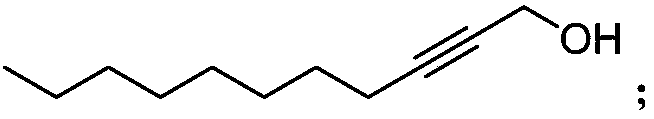
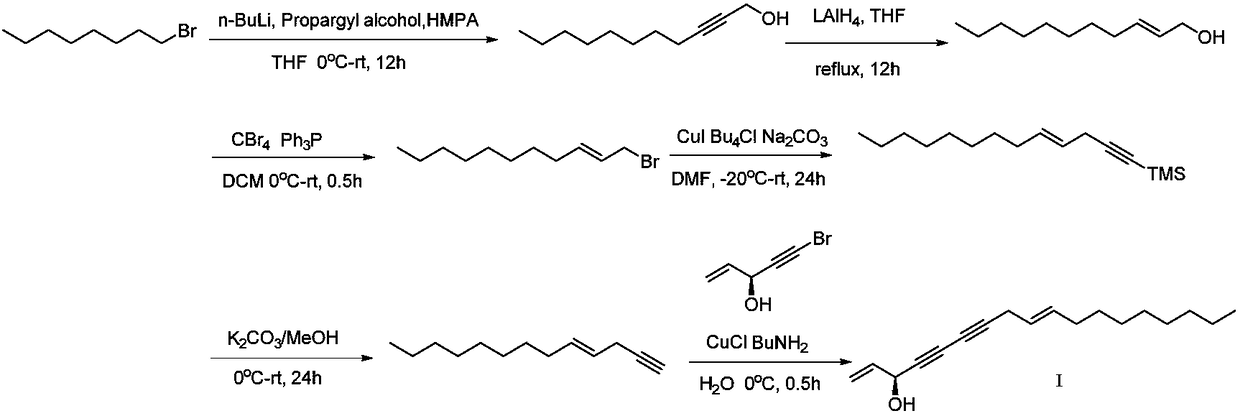
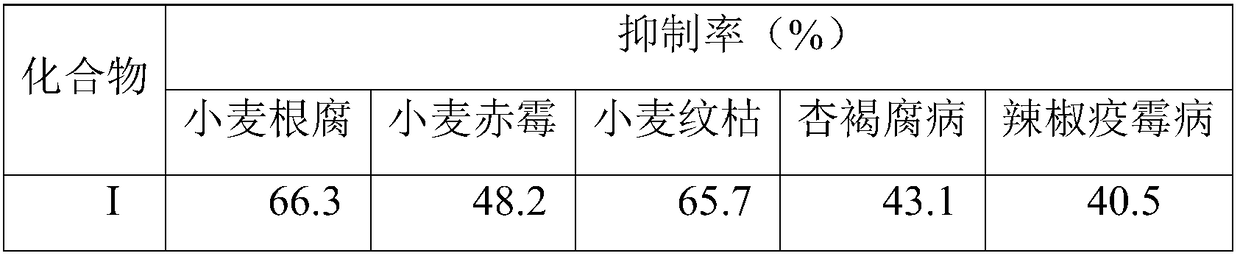
Synthetic method of highly-activity chiral acetylenic alcohol (S,E)-1,9-diene-4,6-diacetyl-3-octadecyl alcohol
A synthesis method, technology of stearyl alcohol, applied in the fields of compounds of group 4/14 elements of the periodic table, organic chemical methods, chemical instruments and methods, etc., can solve the problems of limitations and disadvantages of the three-seventh distribution of the screen, and avoid Effects of racemization, increased yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis method of the highly active chiral acetylenic alcohol (S,E)-1,9-diene-4,6-diyne-3-octadecyl alcohol in this example, the steps are as follows:
[0027] (1) Synthesis of 2-undecyne-1-alcohol: In a 250ml three-necked flask equipped with a magnetic stirrer under inert gas protection, add propynyl alcohol (4.7ml, 80mmol) and hexamethylphosphoryl in sequence Triamine (42ml, 240mmol) and tetrahydrofuran (40ml) were cooled to -78°C, and n-butyllithium (64ml, 160mmol) was added slowly. Stir at -20°C for 3h, then slowly add 1-bromooctane (7.7248g, 40mmol) at -20°C. After the reaction system continued to stir for 50 min, it was heated to room temperature and stirred for 24 h, and the reaction progress was monitored at any time by thin-layer chromatography. After the reaction, the system was quenched with water, the aqueous phase was extracted with ether, the organic phases were combined, washed with saturated saline solution, anhydrous Na 2 SO 4 dry. After preci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com