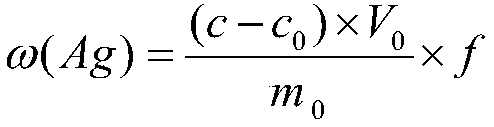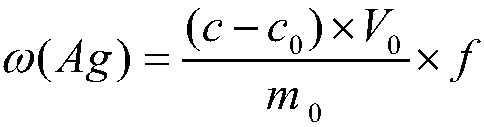Method for determining silver content in crude copper
A technology with silver content and blister copper, applied in the field of analytical chemistry, can solve problems such as labor consumption, time-consuming, environmental pollution, etc., and achieve the effects of simple experimental steps, environmental protection, and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
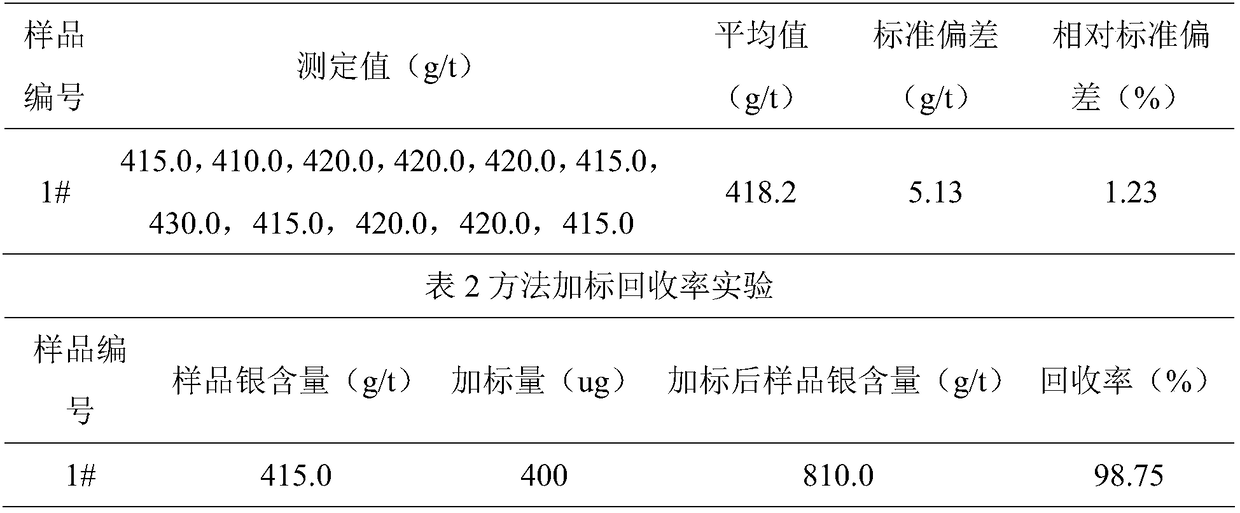
[0026] Weigh the mass of the sample as 1.0000g, denoted as m 0 , accurate to 0.0001g, put the sample in a 250mL beaker, add 1mL water to wet the sample;
[0027] Step 2: Add 20mL (1+1) inverse aqua regia, cover the watch glass, heat and dissolve to 3mL on an electric hob at 220°C, remove and cool to room temperature;
[0028] Step 3, add 5mL of hydrochloric acid, continue heating to drive out the nitric acid, repeat twice;
[0029] Step 4. Transfer the sample solution to a 100mL volumetric flask, add 10-20mL hydrochloric acid, dilute to the mark with deionized water, and record it as V 0 , mixed, static clarified, accompanied by reagent blank;
[0030] Step 5, preparing silver standard series of different concentrations;
[0031] Step 6, dilute the sample solution 5 times, denoted as f, at the wavelength 328.1nm of the atomic absorption spectrophotometer, use the air-acetylene flame, measure the absorbance of the solution, find out the corresponding silver concentration fro...
Embodiment 2
[0049] Step 1. Weigh the mass of the sample as 1.0000g, denoted as m 0 , accurate to 0.0001g, put the sample in a 250mL beaker, add 1.5mL water to wet the sample;
[0050] Step 2: Add 25mL (1+1) inverse aqua regia, cover the watch glass, heat and dissolve on an electric hob at 230°C to 4mL, remove and cool to room temperature;
[0051] Step 3, add 8mL of hydrochloric acid, continue heating to drive out the nitric acid, repeat twice;
[0052] Step 4. Transfer the sample solution to a 100mL volumetric flask, add 15mL hydrochloric acid, dilute to the mark with deionized water, and record it as V 0 , mix well, stand still and clarify, along with the reagent blank;
[0053] Step 5, preparing silver standard series of different concentrations;
[0054] Step 6, dilute the sample solution 10 times, denoted as f, at the wavelength 328.1nm of the atomic absorption spectrophotometer, use the air-acetylene flame, measure the absorbance of the solution, find out the corresponding silver...
Embodiment 3
[0074] Step 1. Weigh the mass of the sample as 1.0000g, denoted as m 0 , accurate to 0.0001g, put the sample in a 250mL beaker, add 2mL water to wet the sample;
[0075] Step 2. Add 30mL (1+1) inverse aqua regia, cover with a watch glass, cover the watch glass, heat and dissolve on an electric stove at 250°C to 5mL, remove and cool to room temperature;
[0076] Step 3, add 10mL of hydrochloric acid, continue heating to drive out the nitric acid, repeat twice;
[0077] Step 4. Transfer the sample solution to a 100mL volumetric flask, add 20mL hydrochloric acid, dilute to the mark with deionized water, and record it as V 0 , mixed, static clarified, accompanied by reagent blank;
[0078] Step 5, preparing silver standard series of different concentrations;
[0079] Step 6, dilute the sample solution 20 times, denoted as f, at the wavelength 328.1nm of the atomic absorption spectrophotometer, use the air-acetylene flame, measure the absorbance of the solution, find out the cor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com