Method for detecting iron content in water sample by using methyl-hydroxamic acid spectrophotometric method
A technology of methylhydroxime and iron content, applied in the measurement of color/spectral characteristics, etc., can solve the problem of low selectivity, and achieve the effect of good selectivity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A methyl hydroxamic acid photometric method for detecting iron content in a water sample includes the following steps:
[0026] 1) Fe 3+ Preparation of standard solution: add Fe 3+ The stock solution is diluted with distilled water to 10.0μg / mL Fe 3+ standard solution;
[0027] 2) Determination of absorbance: Pipette 0.02 ml, 0.40 ml, 0.80 ml, 1.20 ml, 1.60 ml, 2.00 ml, 2.40 ml of 10.0μg / mL Fe respectively 3+ Standard solution in a 25ml volumetric flask, add 2.0ml of 0.207mol / L methyl hydroxamic acid solution, 1ml of 1+1 hydrochloric acid, 5.0ml of aminoacetic acid-hydrochloric acid buffer with pH 2.3, 5.0ml of 3% H 2 O 2 , Shake well, dilute to 25ml with distilled water, take a 1cm cuvette, and measure the corresponding absorbance at the maximum absorption wavelength of 480nm with the reagent blank as a reference;
[0028] 3) Draw the standard curve: draw the standard curve with iron content as the abscissa and the corresponding absorbance as the ordinate, and use one-variabl...
Embodiment 2
[0031] Example 2 Test analysis
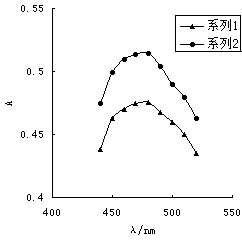
[0032] 2.1 Absorption curve
[0033] Take 2.0 ml Fe 3+ Standard solution, refer to the experimental method in Example 1, measure the absorbance every 10nm between 400 and 600nm, draw the absorption curve, see figure 1 Shown. Of which: Series 1-Fe 3+ +Methyl hydroxamic acid system, series 2-Fe 3+ +Methyl Hydroxamic Acid+H 2 O 2 system.
[0034] by figure 1 It can be seen that 3.0% H 2 O 2 Join Fe 3+ -After methyl hydroxamic acid system, its maximum absorption wavelength is still at 480nm, no red shift occurs, but its absorbance value increases, thus improving the sensitivity of the color reaction, and H 2 O 2 The addition of Chromium greatly increases the stability of the color-developing system, which can make the color-developing system stand for about a week without fading.
[0035] The influence of acidity on color reaction
[0036] Take 2.0 ml Fe 3+ Standard solution, the method refers to Example 1, adjust the pH value of the buffer solution to f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Maximum absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com