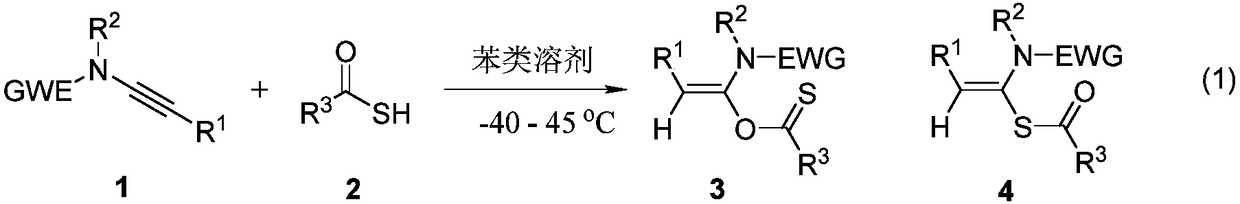
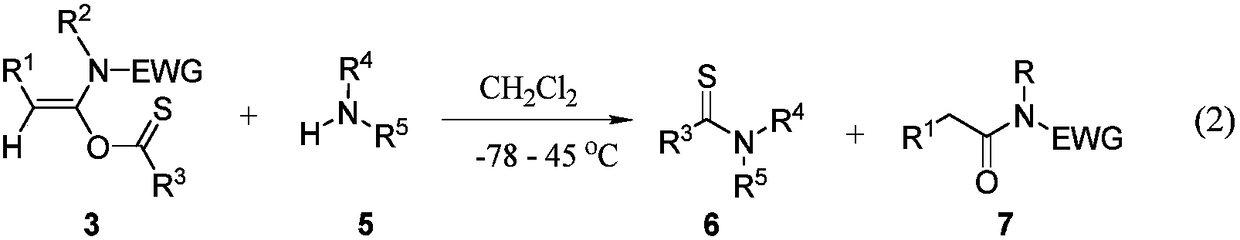
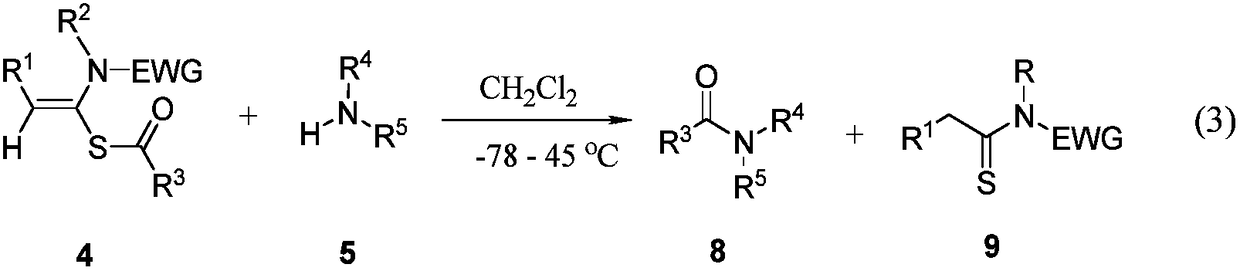
Preparation method of acetylene amide mediated thioacid amide and application of acetylene amide mediated thioacid amide in thiopeptide synthesis
A technology of thioamide and alkyne amide is applied in the preparation of thioamide mediated by alkyne amide and its application in the synthesis of thiopeptides, achieving broad scientific research and industrial application prospects, mild synthesis, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add N-methylyne-p-toluenesulfonamide (0.24mmol) and thioacetic acid (0.20mmol) to a clean 25mL reaction tube, add an appropriate amount of dichloromethane as a solvent, react at room temperature for 5min, and detect by TLC spotting. Solvent concentration and column chromatography gave pure products as yellow liquid a1 (53% yield) and white solid a2 (46% yield). The following are the structural formula and NMR experimental data of the product:
[0042]
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.65(d, J=8.0Hz, 2H), 7.26(d, J=8.0Hz, 2H), 4.72 (d, J=2.8Hz, 1H), 4.54(d, J=2.8Hz, 1H) ,2.99(s,3H),2.50(s,3H),2.37(s,3H); 13 C NMR (100MHz, CDCl 3 )δ217.5, 150.2, 144.3, 133.5, 129.6, 128.0, 100.4, 38.2, 34.0, 21.6ppm.
[0044]
[0045] 1 H NMR (400MHz, CDCl 3 )δ7.58(d, J=8.0Hz, 2H), 7.25(d, J=8.0Hz, 2H), 5.84(s, 1H), 5.55(s, 1H), 2.90(s, 3H), 2.38( s,3H),2.22(s,3H); 13 C NMR (100MHz, CDCl 3 )δ192.9, 144.1, 135.4, 134.3, 130.1, 129.6, 127.9, 36.4, 30.2, 21.6ppm.
Embodiment 2
[0047] Add N-methylyne-p-toluenesulfonamide (0.24mmol) and thiobenzoic acid (0.20mmol) into a clean 25mL reaction tube, add an appropriate amount of dichloromethane as a solvent, react at room temperature for 5min, detect by TLC, and the reaction is over Afterwards, the solvent was concentrated and subjected to column chromatography to obtain pure products, yellow liquid b1 (yield 55%) and colorless liquid b2 (yield 43%). The following are the structural formula and NMR experimental data of the product:
[0048]
[0049] 1 H NMR (400MHz, CDCl 3 )δ8.10(dd, J=8.5,1.2Hz,2H),7.72(d,J=8.3Hz,2H),7.56(ddt,J=8.7,7.6,1.2Hz,1H),7.36(t,J =7.9Hz,2H),7.25(d,J=7.9Hz,2H),4.93(d,J=2.9Hz,1H),4.81(d,J=2.8Hz,1H),3.12(s,3H), 2.39(s,3H); 13 C NMR (100MHz, CDCl 3 )δ208.6, 150.1, 144.2, 137.4, 133.8, 133.4, 129.5, 129.4, 128.2, 128.1, 101.5, 38.1, 21.6ppm.
[0050]
[0051] 1 H NMR (400MHz, CDCl 3 )δ7.83(dd, J=8.3,1.3Hz,2H),7.70(d,J=8.3Hz,2H),7.59(t,J=7.1Hz,1H),7.44(t,J=7.9Hz, 2H), 7....
Embodiment 3
[0053] Add N-methylyne-p-toluenesulfonamide (0.24mmol) and p-chlorothiobenzoic acid (0.20 mmol) into a clean 25mL reaction tube, add an appropriate amount of dichloromethane as a solvent, react at room temperature for 5min, and detect by TLC , after the reaction, the solvent was concentrated and column chromatography was used to obtain pure products, yellow solid c1 (yield 57%) and yellow solid c2 (yield 41%). The following are the structural formula and NMR experimental data of the product:
[0054]
[0055] 1 H NMR (400MHz, CDCl 3 )δ8.06(d, J=8.7Hz, 2H), 7.71(d, J=8.3Hz, 2H), 7.34 (d, J=8.7Hz, 2H), 7.27(d, J=8.2Hz, 2H) ,4.92(d,J=2.9Hz,1H),4.75(d,J=2.8Hz,1H),3.11(s,3H),2.41(s,3H); 13 C NMR (100MHz, CDCl 3 )δ206.9, 150.2, 144.2, 140.1, 135.9, 133.6, 130.7, 129.5, 128.4, 128.1, 101.3, 38.3, 21.6ppm.
[0056]
[0057] 1 H NMR (400MHz, CDCl 3 )δ7.77(d, J=8.6Hz, 2H), 7.69(d, J=8.2Hz, 2H), 7.41(d, J=8.6Hz, 2H), 7.29(d, J=8.0Hz, 2H) ,5.96(s,1H),5.74(s,1H),3.07(s,3H),2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com