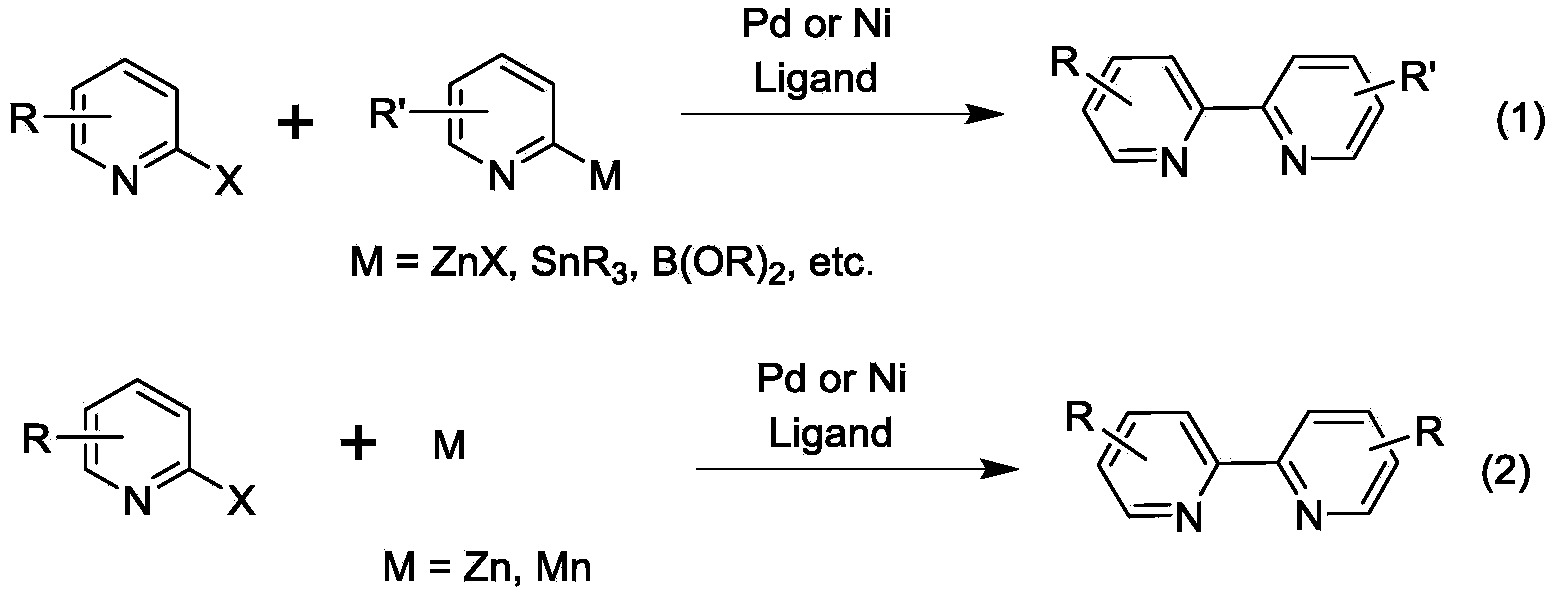
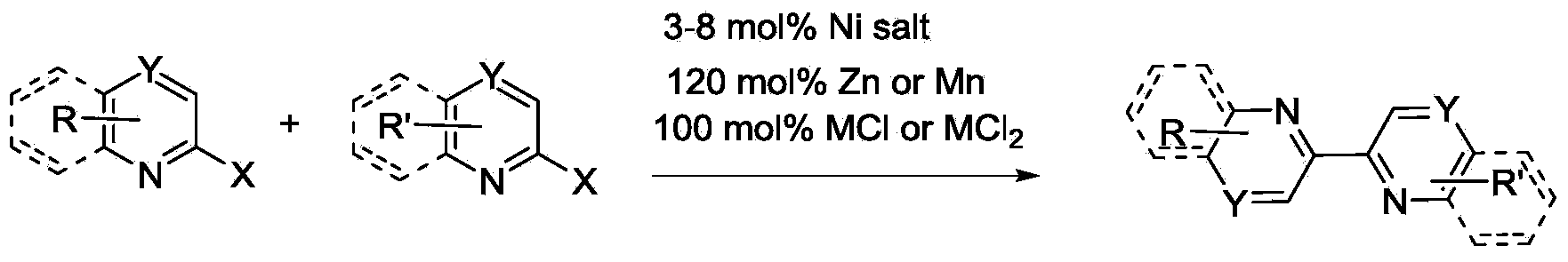
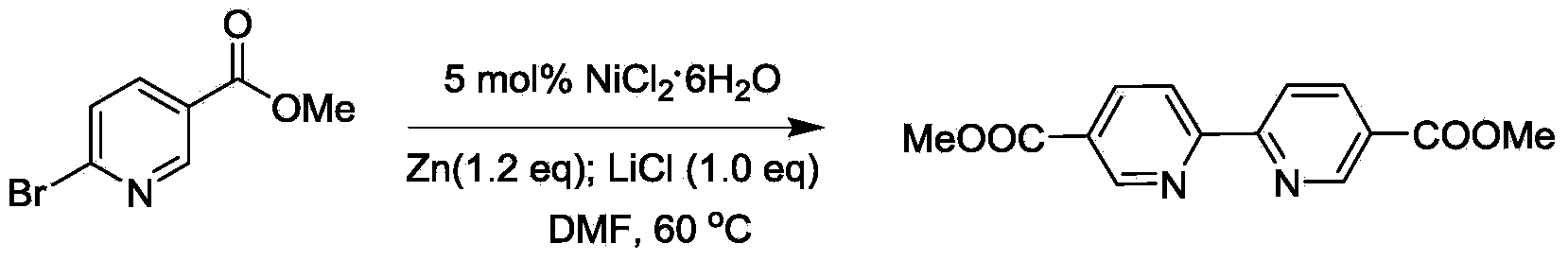Synthesis method of dipyridyl derivative or analogue
A synthesis method and derivative technology, applied in the field of bipyridine derivatives and analogues, can solve the problems of unsuitable industrial production, waste of reagents, environment, retention, etc., and achieve the effects of high selectivity, wide application range, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, the synthesis of 2,2'-dipyridine-5,5'-methyl dicarboxylate
[0028]
[0029] Add nickel chloride hexahydrate (0.12g, 0.5mmol) into 20mL N,N-dimethylformamide, add 2-bromopyridine-5-carboxylic acid methyl ester (2.16g, 10mmol), anhydrous lithium chloride (0.43g, 10mmol) and zinc powder (0.78g, 12mmol). Heat the system at 40°C, add a grain of iodine to initiate the reaction, and then keep it warm at 55-60°C until the reaction is complete. The reaction mixture was neutralized to alkaline by adding concentrated ammonia water, the resulting mixture was extracted with dichloromethane, the organic layer was dried over anhydrous sodium carbonate, and the solvent was recovered, and the obtained crude product was purified by column chromatography to obtain the target product 2,2'-bipyridine- Methyl 5,5'-dicarboxylate, yield: 77%.
[0030] The product is a white solid, mp: 260.6-261.9°C.ν max (KBr) / cm -1 1727. 1 H NMR (400MHz, CDCl 3 ):δ9.23(s,2H),8.52(s,2H)...
Embodiment 2、2
[0031] The synthesis of embodiment 2,2,2'-quinoxaline
[0032]
[0033] Nickel chloride hexahydrate (0.12g, 0.5mmol) was added to 20mL N,N-dimethylformamide, followed by adding 2-chloroquinoxaline (1.65g, 10mmol), anhydrous lithium chloride (0.43g, 10mmol) and manganese powder (0.61g, 12mmol). Heat the system at 40°C, add a grain of iodine to initiate the reaction, and then keep it warm at 55-60°C until the reaction is complete. The reaction mixture was neutralized to alkaline by adding concentrated ammonia water, the resulting mixture was extracted with dichloromethane, the organic layer was dried over anhydrous sodium carbonate, and the solvent was recovered, and the obtained crude product was purified by column chromatography to obtain the target product 2,2'-quinoxaline , Yield: 72%.
[0034] The product is a white solid, mp193-195°C. 1 H NMR (400MHz, CDCl 3 ): δ8.81(d, J=8.6Hz, 2H), 8.28(d, J=8.6Hz, 2H), 8.20(d, J=8.3Hz, 2H), 7.83(d, J=8.1Hz, 2H ), 7.70(t, J=7.2Hz...
Embodiment 3
[0035] Embodiment 3, the synthesis of 2,2'-bipyridine-6,6'-dicarbonitrile
[0036]
[0037] Nickel chloride hexahydrate (0.05g, 0.2mmol) was added to 20mL N,N-dimethylformamide, followed by adding 2-chloro-6-cyanopyridine (1.39g, 10mmol), anhydrous lithium chloride (0.65 g, 15mmol) and zinc powder (0.56g, 11mmol). Heat the system at 40°C, add a grain of iodine to initiate the reaction, and then keep it warm at 55-60°C until the reaction is complete. The reaction mixture was neutralized to alkaline by adding concentrated ammonia water, the resulting mixture was extracted with dichloromethane, the organic layer was dried over anhydrous sodium carbonate, and the solvent was recovered, and the obtained crude product was purified by column chromatography to obtain the target product 2,2'-bipyridine- 6,6'-dicarbonitrile, yield: 83%.
[0038] The product is white solid, mp264.5-265.9℃.ν max (KBr) / cm -1 2236. 1 H NMR (400MHz, CDCl 3 ): δ8.73(d, J=8.1Hz, 2H), 8.02(t, J=7.8Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com