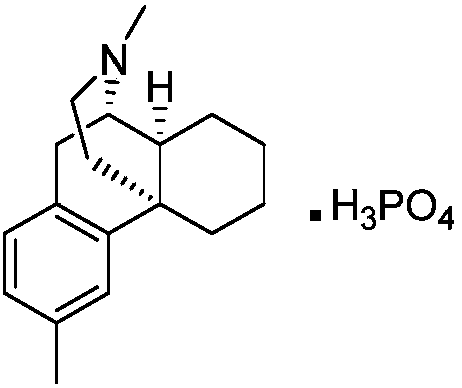
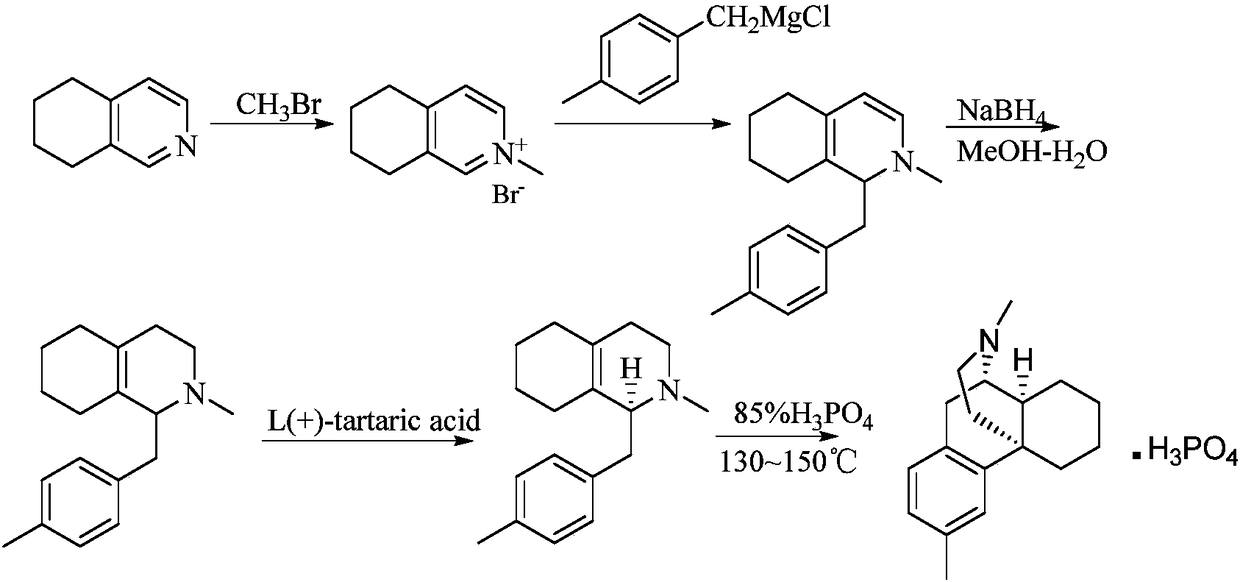
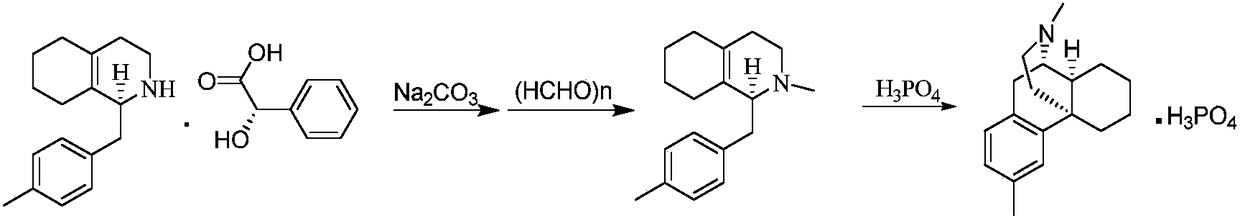
Preparation method of dimemorfan phosphate
A technology of dimethylphene phosphate and phosphoric acid, which is applied in the field of preparing non-addictive central antitussive drug dimethylphene phosphate, can solve the problems of general yield and the like, and achieves high product purity, low cost and concise process route. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Under the protection of nitrogen, the starting material 3-methoxy-17-methyldextromorphan (5.71g, 20mmol) and bis(tricyclohexylphosphine)nickel dichloride (0.70g, 1mmol) were added to 100ml of toluene and stirred Dissolve, heat up to 65°C, add methylmagnesium bromide (tetrahydrofuran solution) (1mol / L, 20ml, 20mmol) dropwise, complete the dropwise addition and keep warm for reaction, TLC detects that the raw materials are cooled to room temperature after the reaction is completed, add dropwise a small amount of saturated chloride Quench the reaction with ammonium solution, add water to wash and separate the layers, and separate the organic layer; wash the organic layer with saturated brine, and then dry it, then distill off the toluene under reduced pressure to obtain dimerphane; without purification, directly add 25ml of 95% ethanol and stir at room temperature to dissolve , and then add 85% phosphoric acid (2.3g, 20mmol) to react and precipitate a white solid, which is ...
Embodiment 2
[0038] Under nitrogen protection, the starting material 3-methoxy-17-methyldextromorphan (5.71g, 20mmol) and bis(tricyclohexylphosphine)nickel dichloride (0.14g, 0.2mmol) were added in 100ml of toluene Stir to dissolve, raise the temperature to 65°C, add methylmagnesium bromide (tetrahydrofuran solution) (1mol / L, 20ml, 20mmol) dropwise, and keep warm for reaction after the dropwise addition, after the reaction is detected by TLC. Subsequent reaction was carried out according to the method of Example 1 to obtain 3.76 g of dimethylorphinyl phosphate, with a yield of 53.3% and a purity of 93.7%.
Embodiment 3
[0040] Under the protection of nitrogen, the starting material 3-methoxy-17-methyldextromorphan (5.71g, 20mmol) and bis(tricyclohexylphosphine)nickel dichloride (0.70g, 1mmol) were added to 100ml of toluene and stirred Dissolve, heat up to 65°C, add methylmagnesium bromide (tetrahydrofuran solution) (1mol / L, 60ml, 60mmol) dropwise, complete the dropwise insulation reaction, after TLC detects that the raw materials have reacted, carry out subsequent reactions according to the method of Example 1 to obtain 5.51 g of dimetyl phosphate, yield 78.1%, purity 97.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com