Preparation and application of long wavelength colorimetric fluorescent probe capable of rapidly and high-selectively analyzing thiophenol
A dinitrobromobenzene and compound technology, applied in the field of long-wavelength colorimetric fluorescent probes, can solve problems such as short excitation and emission wavelengths, insufficient response speed, and insufficient selectivity, and achieve fast reaction speed and stability Good, easy to synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] (Scheme 1) Dissolve 741mg (3mmol) of 2,4-dinitrobromobenzene in 8mL of DMF solution, then add 453mg (1mmol) of FR dye and 650mg (2mmol) of cesium carbonate and stir at room temperature for 10h, then add water to precipitate the solid to obtain crude product. If a purer product is to be obtained, the crude product can be subjected to thin-layer chromatography with pure dichloromethane system to obtain a pure product. 574 mg of pure orange product was obtained with a yield of 82%.
[0032] (Scheme 2) Dissolve 988mg (4mmol) of 2,4-dinitrobromobenzene in 8mL of DMF solution, then add 453mg (1mmol) of FR dye and 650mg (2mmol) of cesium carbonate and stir at room temperature for 10h, then add water to precipitate the solid to obtain crude product. If a purer product is to be obtained, the crude product can be subjected to thin-layer chromatography with pure dichloromethane system to obtain a pure product. Obtained 490mg of orange pure product, the yield was 70%...
Embodiment 2
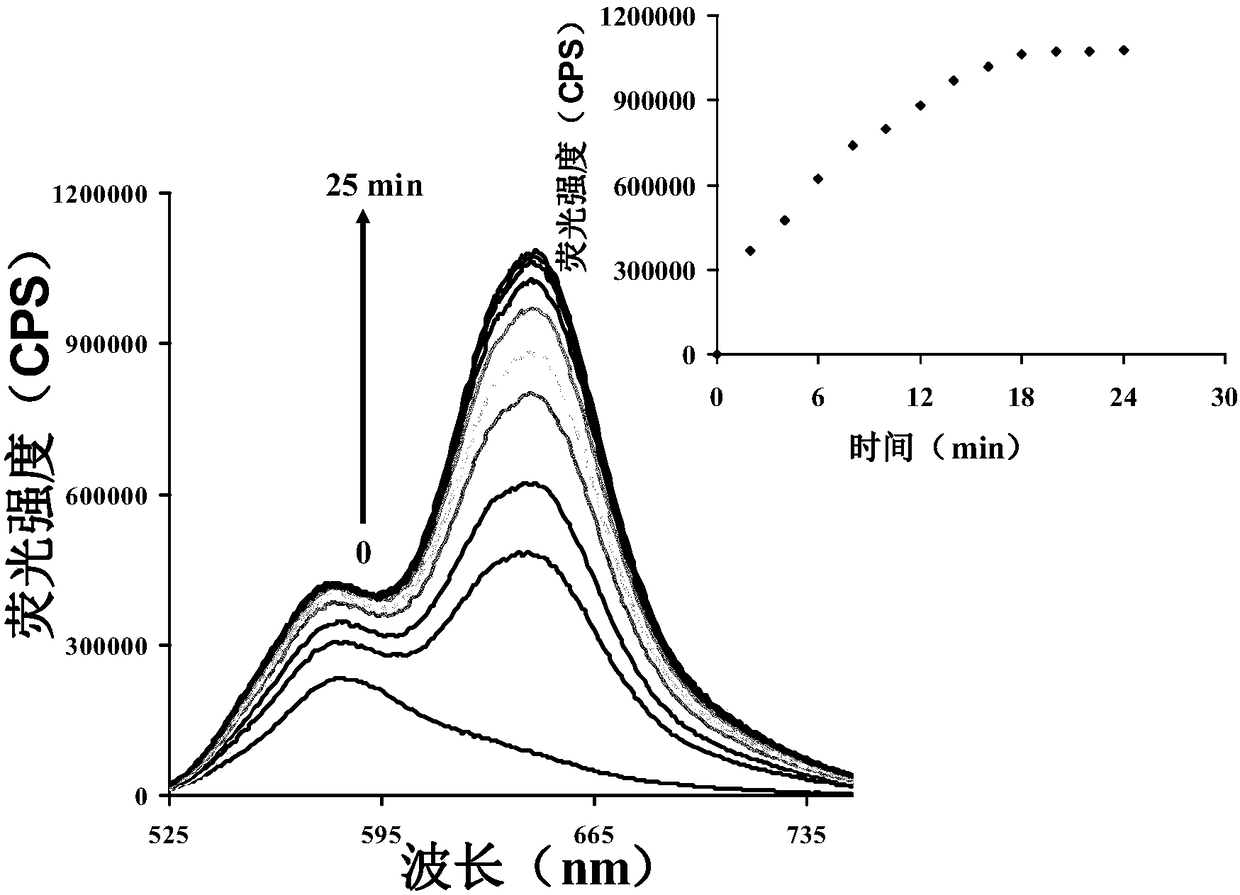
[0037] figure 1 It is the fluorescence spectrum of the probe (5μM) before and after adding thiophenol (50μM). We can see that the fluorescence change is very obvious through the illustration.
Embodiment 3
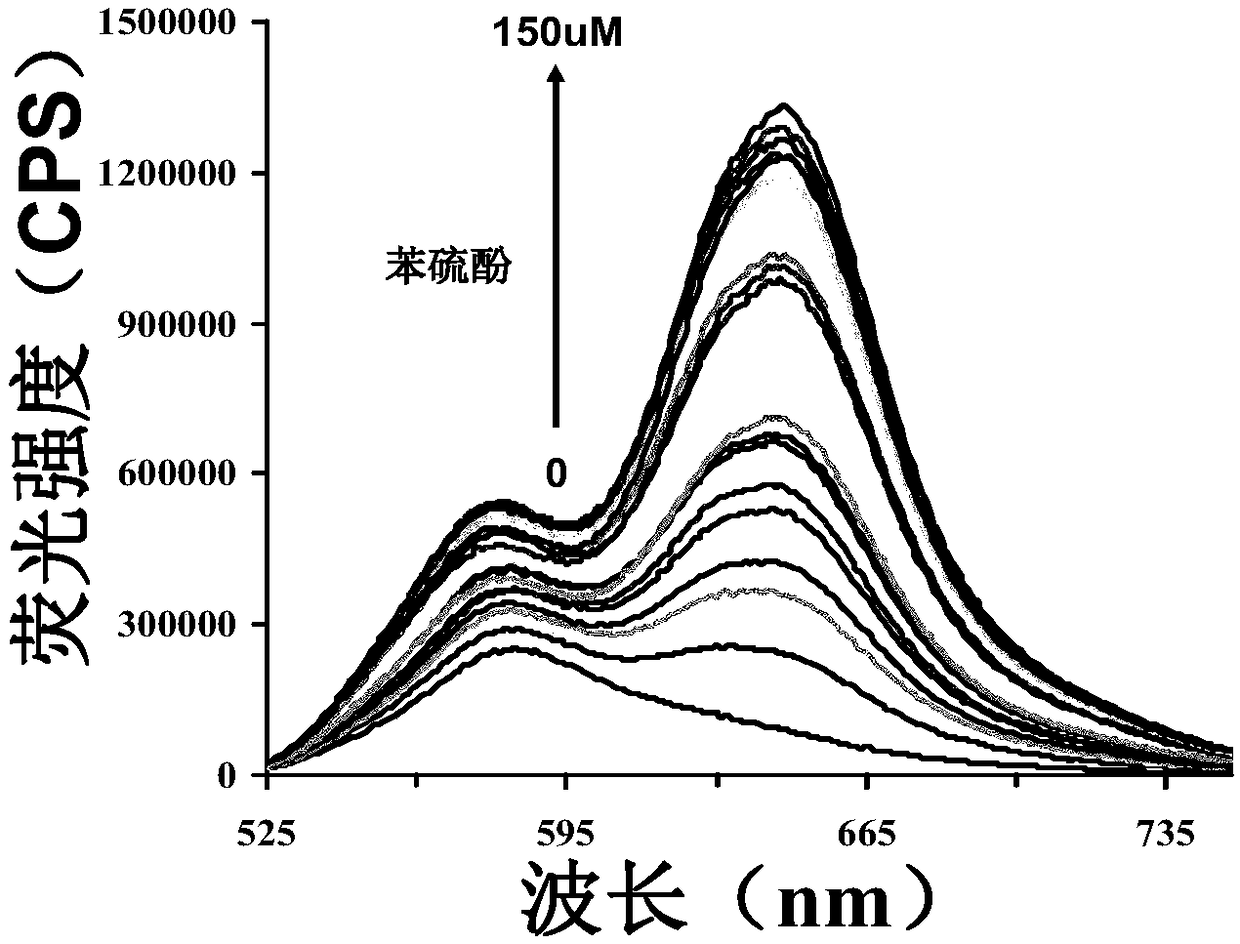
[0039] Figure 2a is the effect of different concentrations of thiophenol (0-150μM) on the fluorescence intensity of the probe (5μM).
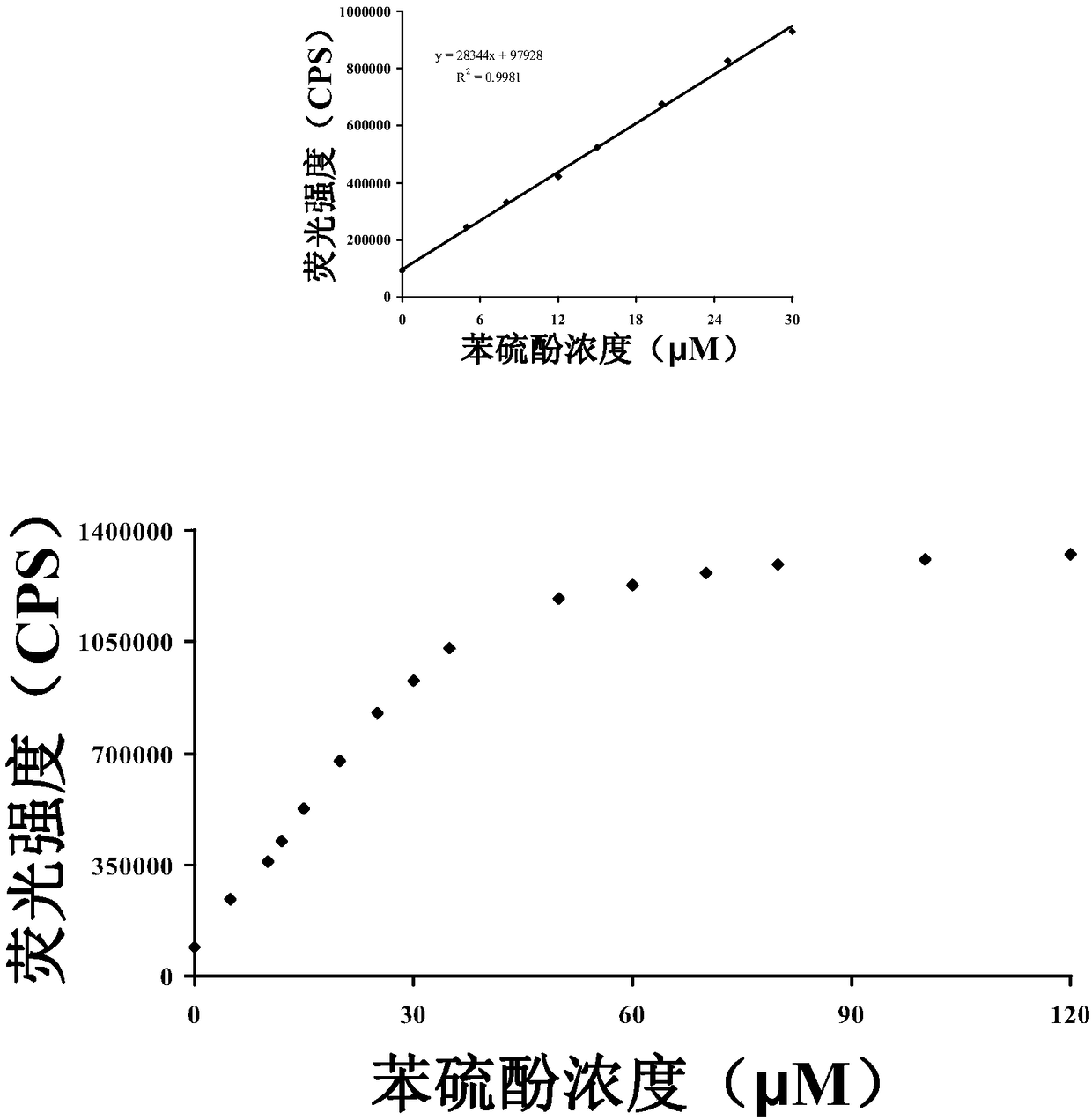
[0040] Figure 2b It can be seen that within the concentration range of (0-30 μM) thiophenol, the concentration of thiophenol has a linear relationship with the fluorescence intensity, and the fluorescence intensity gradually remains unchanged as the concentration of thiophenol in the probe solution continues to increase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com