Synthesis of multi-signal fluorescence probe and application of multi-signal fluorescence probe in simultaneously and differentially detecting Hcy, Cys and GSH (Glutathione)
A technology of fluorescent probes and fluorescent molecular probes, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Synthesis of 9-hydroxy-2,3,6,7-tetrahydro-1H,5H,11H pyrano[2,3-F]pyrido[3,2,1-ij]quinoline-11 -ketone
[0027] a. Add 16.3 g (63.4 mmol) of diphenyl malonate and 10.0 g (52.8 mmol) of 8-hydroxyjuloridine to 100 mL of anhydrous toluene, and stir the reaction at 100 °C for 8-12 hours,
[0028]b. After the reaction was completed, the reaction solution was cooled to room temperature and filtered, the filter cake was washed with diethyl ether, and the obtained solid was vacuum-dried to obtain a gray-green solid 9-hydroxy-2,3,6,7-tetrahydro-1H, 5H, 11H pyrano[2,3-F]pyrido[3,2,1-ij]quinolin-11-one 11.0 g, yield 80.9%.
Embodiment 2
[0029] Example 2. Synthesis of 9-chloro-11-oxo-2,3,6,7-tetrahydro-1H,5H,11H pyrano[2,3-F]pyrido[3,2,1-ij ]quinoline-10-carbaldehyde
[0030] . Under nitrogen protection, 18 mL of dry re-distilled N,N-dimethylformamide (DMF) was slowly added to an equal volume of phosphorus oxychloride (POCl 3 ), stirring at 20-50 °C for 30 minutes to obtain a red solution,
[0031] . 15.0 g (58.3 mmol) of 9-hydroxy-2,3,6,7-tetrahydro-1H,5H,11H pyrano[2,3-F]pyrido[3,2,1-ij]quinoline -11-keto was dissolved in 70 mL N,N-dimethylformamide and added dropwise to step In the mixed solution, the mixture continued to stir and react at 60°C for 12 hours under nitrogen protection;
[0032] . After the reaction is complete, the steps The reaction solution in the solution was slowly poured into 500 mL of ice water, and the pH was adjusted to 6 with 20% NaOH solution, resulting in a large amount of precipitation, filtered, and the filter cake was washed 3 times with an appropriate amount of d...
Embodiment 3
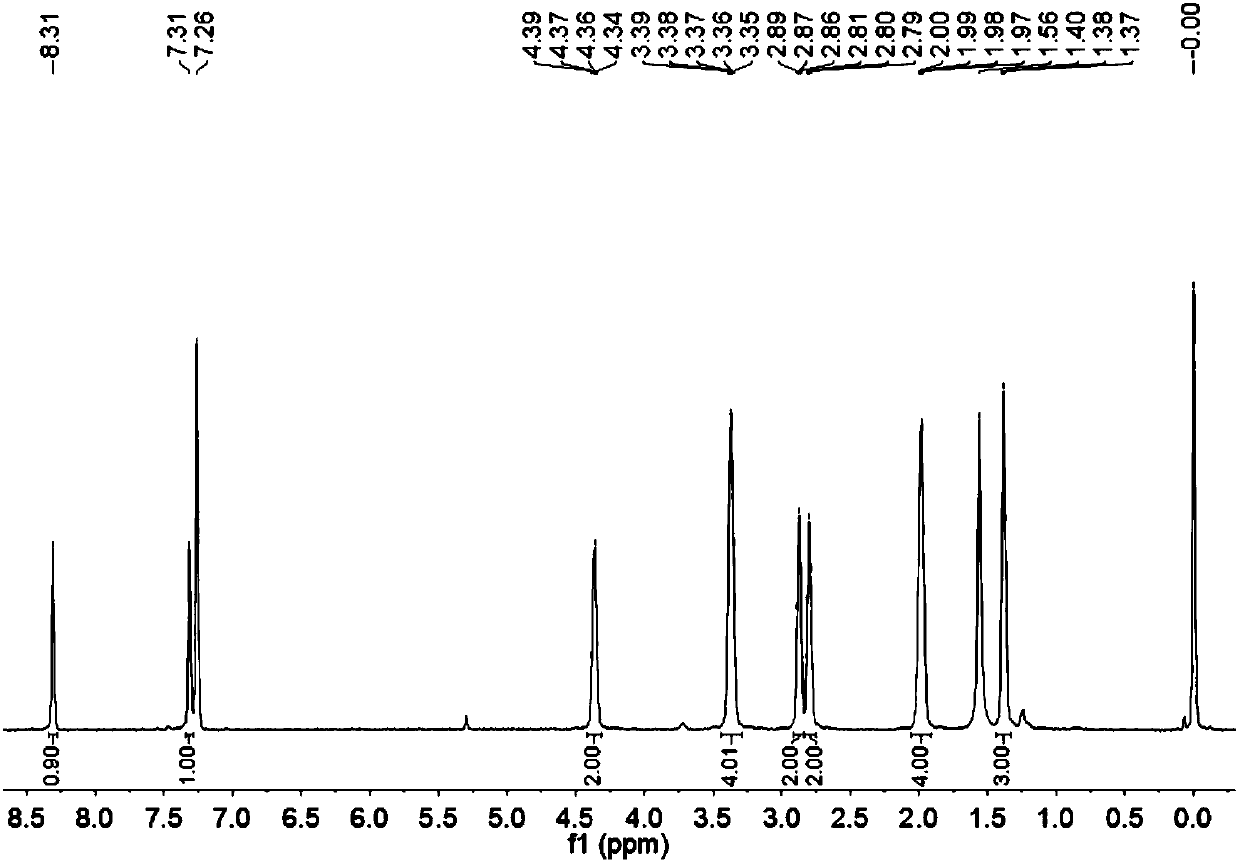
[0033] Example 3. Synthesis of multi-signal fluorescent probes ( E )-3-(9-chloro-11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1- ij]quinolin-10-yl)-2-cyanoacrylate ethyl ester (R = ethyl)
[0034] . 5.0 g (16.5 mmol) of 9-chloro-11-oxo-2,3,6,7-tetrahydro-1H,5H,11H pyrano[2,3-F]pyrido[3,2, 1-ij]quinoline-10-carbaldehyde and 2.79 g (24.7 mmol) ethyl cyanoacetate were mixed and added to 30 mL of anhydrous dichloromethane, then 0.2 mL of triethylamine was added dropwise, and the reaction was stirred at room temperature;
[0035] . After the reaction is complete, step The reaction droplets were added to 300 mL of absolute ethanol, filtered, and the obtained solid was dried to obtain the fluorescent molecular probe according to claim 1 ( E )-3-(9-chloro-11-oxo-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1- ij]quinolin-10-yl)-2-cyanoacrylate ethyl ester 4.5 g, yield 68.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com