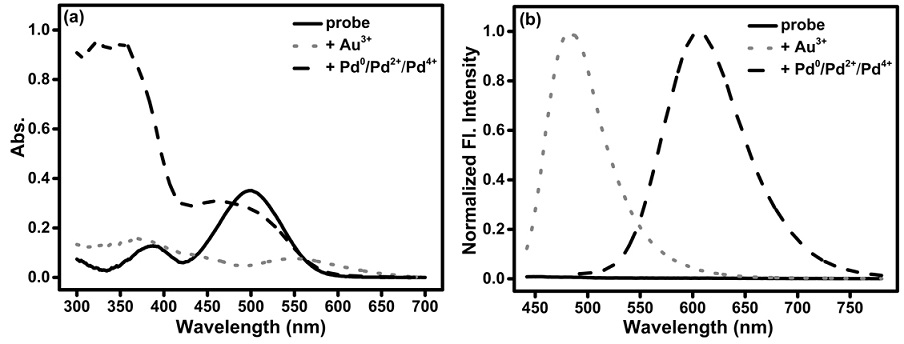
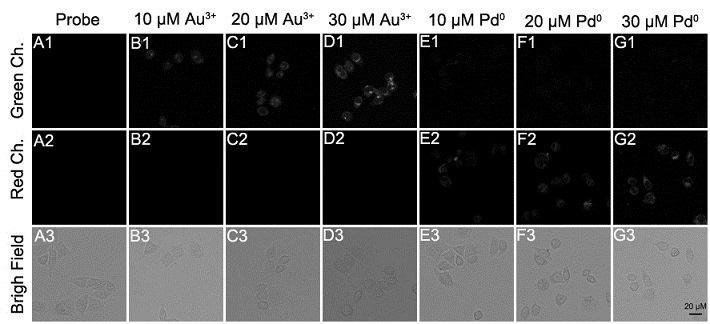
Synthesis and application of a fluorescent probe for simultaneously distinguishing between gold ions au3+ and palladium
A fluorescent probe, gold ion technology, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Synthesis of 5-ethyl-8-methoxy-1,2,3,3a,4,5-hexahydropyrrololo (1,2-a) quinoxaline
[0028] a. 30g (175.31mmol) 3-fluoro-4-nitroanisole and 37.5g (227.9mmol) L-proline methyl ester hydrochloride was added to 200mL anhydrous acetonitrile, and then added 73mL (525.9mmol) triethylamine heated reflux reaction for 6 hours, filtered to remove triethylamine hydrochloride, spin-drying solution,
[0029] b. Dissolve the drying solution with 300mL methanol, slowly add 46.76g (874.13mmol) ammonium chloride and 57.15g (874.13mmol) zinc powder, stir overnight at room temperature, and then filter to remove the zinc powder, spin out the solvent, add the solid to the water, filter the green solid,
[0030] c. The green solid was dissolved with 400 mL anhydrous tetrahydrofuran, 36.4 g (962.17 mmol) sodium borohydride and 122 mL (962.17 mmol) boron trifluoride diethyl ether were added, the reaction was completed for 12 hours, after the reaction was complete, slowly poured into the...
Embodiment 2
[0032] Example 2. Synthesis of 5-ethyl-8-hydroxy-1,2,3,3a,4,5-hexahydropyrrole [1,2-a]quinoxaline-7-carboxaldehyde
[0033] I. Under nitrogen protection conditions, an appropriate amount of drying and re-distilled 10mL N, N-dimethylformamide (DMF) was slowly added to 10mL (64.22mmol) phosphorus chloride (POCl). 3) in, stirred at 20-50 ° C for 30-60 minutes to give a yellow solution, 10g (35.68mmol) 5-ethyl-8-methoxy-1,2,3a,4,5-hexahydropyrrole (1,2-a) quinoxaline dissolved in 40mL N, N-dimethylformamide (DMF), dropwise added to the yellow mixed solution, the mixture continued under nitrogen protection at 60 °C stirring reaction for 12 hours; After the reaction is complete, the reaction solution is poured into an appropriate amount of ice water, adjust the pH to 5 to 6 with 20% NaOH solution, and then extracted with ethyl acetate, the organic layer is dried to give a yellow solution,
[0034] II. 621.8mg (23.05mmol) aluminum and 5.85g (23.05mmol) iodine added to 15mL of anhydrous a...
Embodiment 3
[0035] Example 3. Synthesis of 2- ((5-ethyl-8-(propyl-2-ayn-1-yloxy)-1,2,3,3a,4,5-hexahydropyrrolofa [1,2-a]quinoxa-7-yl) methylene) malonitrile
[0036] i. 200mg (811.9 μmol) 5-ethyl-8-hydroxy-1,2,3,3a,4,5-hexahydropyrrole [1,2-a]quinoxaline-7-carboxaldehyde and 289mg (2.44mmol) acryloyl chloride was added to 8mL of anhydrous dichloromethane, and then 246.5mg (2.44mmol) was added to re-distill triethylamine, and the reaction at room temperature was 3 hours, and the cyan solid was obtained by the column 140mg, with a yield of 60.63%;
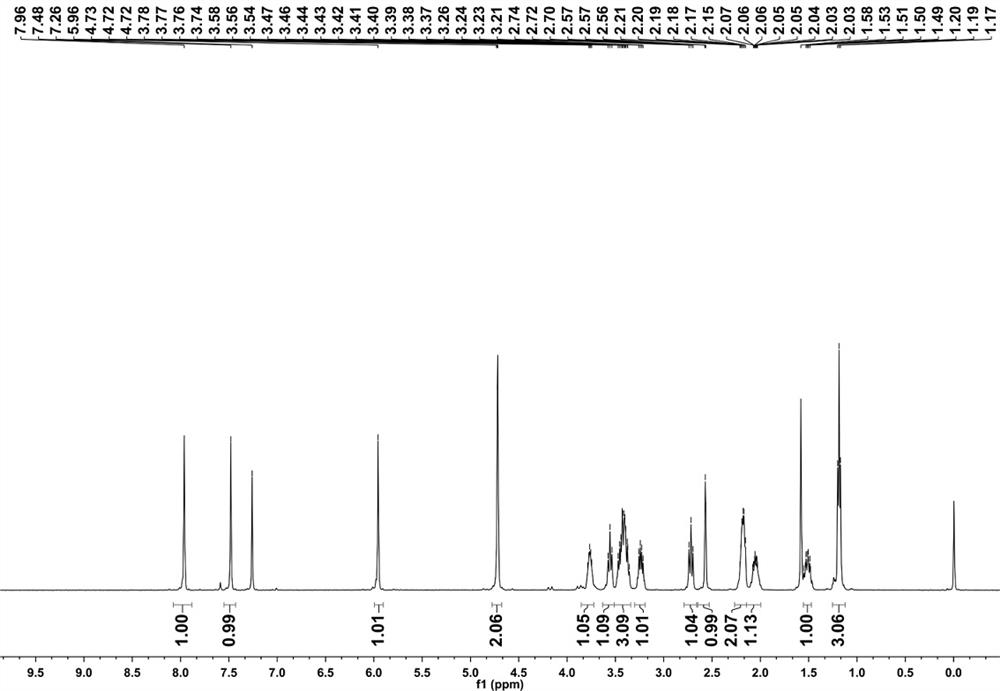
[0037] ii. 140mg (492.3 μmol) solid and 39.02mg (590.80 μmol) malonitrile in step i was added to 8 mL ethanol, reacted overnight at room temperature, filtered to obtain the fluorescent molecular probe 2- (5-ethyl-8-(prop-2-alkyn-1-yloxy) -1,2,3a,4,5-hexahydropyrrole [1,2-a] quinoxaline-7-yl) methylene) malonitrile 101mg, yield 61.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com