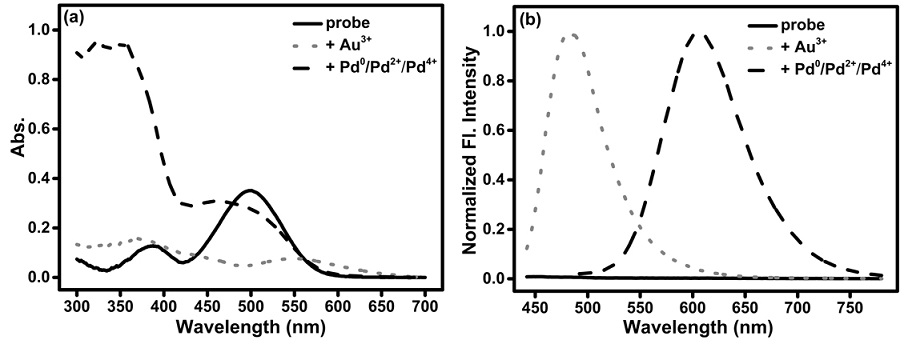
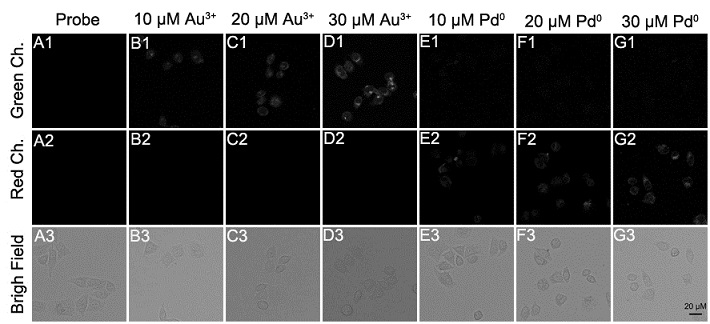
Synthesis and application of fluorescent probe for simultaneously distinguishing gold ions and palladium species
A technology of fluorescent probes and fluorescent molecular probes, applied in the synthesis and application of fluorescent probes that simultaneously distinguish between gold ions and palladium species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
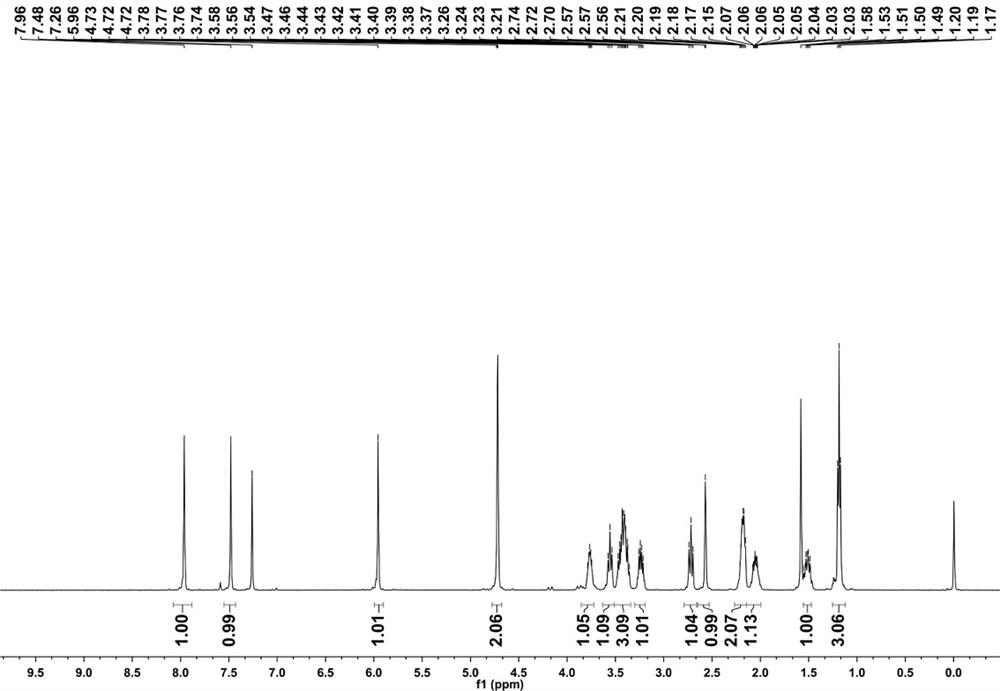
[0027] Example 1. Synthesis of 5-ethyl-8-methoxy-1,2,3,3a,4,5-hexahydropyrrolo(1,2-a)quinoxaline
[0028] a. Add 30 g (175.31 mmol) 3-fluoro-4-nitroanisole and 37.5 g (227.9 mmol) L-proline methyl ester hydrochloride to 200 mL of anhydrous acetonitrile, then add 73 mL ( 525.9 mmol) triethylamine heating and reflux reaction for 6 hours, filtered to remove triethylamine hydrochloride, spin-dried solution,
[0029] b. Dissolve the spin-dried solution with 300 mL of methanol, slowly add 46.76 g (874.13 mmol) of ammonium chloride and 57.15 g (874.13 mmol) of zinc powder, stir overnight at room temperature, then filter to remove the zinc powder, spin to dry the solvent, The solid was added to water and filtered to obtain a green solid,
[0030] c. Dissolve the green solid in 400 mL of anhydrous tetrahydrofuran, add 36.4 g (962.17 mmol) of sodium borohydride and 122 mL (962.17 mmol) of boron trifluoride ether, and react at 90°C for 12 hours. After the reaction is complete, slowly po...
Embodiment 2
[0032] Example 2. Synthesis of 5-ethyl-8-hydroxyl-1,2,3,3a,4,5-hexahydropyrrolo[1,2-a]quinoxaline-7-carbaldehyde
[0033] . Under nitrogen protection, add 10 mL (64.22 mmol) of phosphorus oxychloride (POCl 3 ), stirred at 20-50°C for 30-60 minutes to obtain a yellow solution, and 10 g (35.68 mmol) of 5-ethyl-8-methoxy-1,2,3a,4,5-hexahydropyrrole And (1,2-a) quinoxaline was dissolved in 40mL N,N-dimethylformamide (DMF), and added dropwise to the yellow mixed solution, and the mixture continued to stir and react at 60°C under nitrogen protection for 12 hours; After the reaction was complete, the reaction solution was poured into an appropriate amount of ice water, and the pH was adjusted to 5-6 with 20% NaOH solution, then extracted with ethyl acetate, and the organic layer was spin-dried to obtain a yellow solution.
[0034] . Add 621.8 mg (23.05 mmol) of aluminum and 5.85 g (23.05 mmol) of iodine into 15 mL of anhydrous acetonitrile, react for 10 minutes, dissolve the a...
Embodiment 3
[0035] Example 3. Synthesis of 2-((5-ethyl-8-(prop-2-yn-1-yloxy)-1,2,3,3a,4,5-hexahydropyrrolo[1,2 -a]quinoxal-7-yl)methylene)malononitrile
[0036] . 200 mg (811.9μmol) 5-ethyl-8-hydroxyl-1,2,3,3a,4,5-hexahydropyrrolo[1,2-a]quinoxaline-7-carbaldehyde and 289 mg (2.44 mmol) acryloyl chloride was added to 8 mL of anhydrous dichloromethane, then 246.5 mg (2.44 mmol) redistilled triethylamine was added, reacted at room temperature for 3 hours, passed through the column to obtain 140 mg of a cyan solid, and the yield was 60.63%;
[0037] . Step 140 mg (492.3 μmol) of solid and 39.02 mg (590.80 μmol) of malononitrile were added to 8 mL of ethanol, reacted overnight at room temperature, and filtered to obtain the fluorescent molecular probe 2-((5-ethyl-8- (prop-2-yn-1-yloxy)-1,2,3,3a,4,5-hexahydropyrrolo[1,2-a]quinoxal-7-yl)methylene)propanedi Nitrile 101mg, yield 61.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com