Tetraphenylethylene-based subcellular organelle viscosity probe, and preparation method and application thereof
A tetraphenylethylene and subcellular technology, applied in chemical instruments and methods, instruments, scientific instruments, etc., can solve the problems of insufficient water solubility of probes and low imaging contrast, and achieve good targeting specificity and strong anti-interference , Good water solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
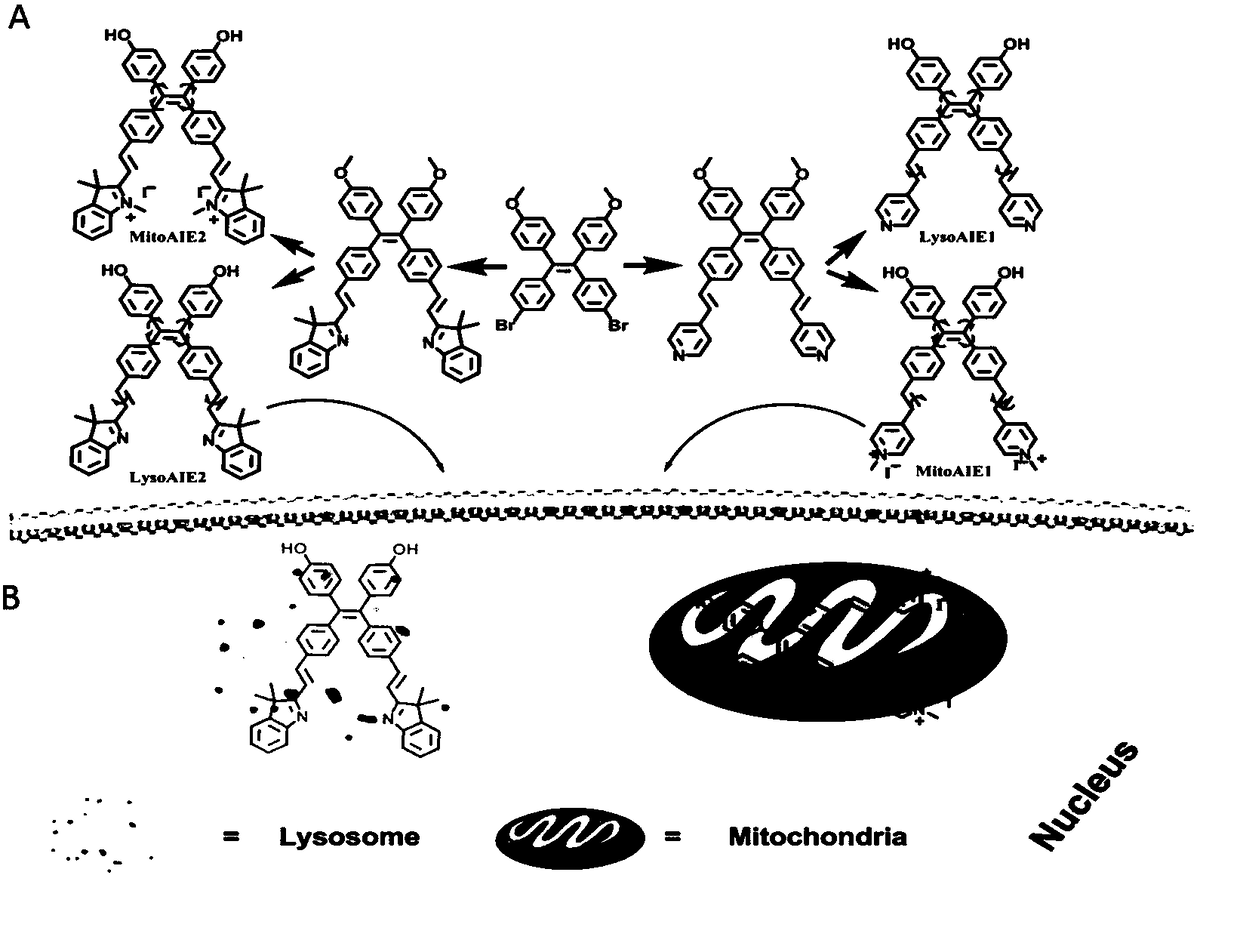
[0065] Tetraphenylethylene-based subcellular organelle viscosity probes, the subcellular organelle viscosity probes include mitochondrial viscosity probes and lysosome viscosity probes, wherein the structural formula of the mitochondrial viscosity probes is as follows:
[0066]
[0067] Wherein the structural formula of the lysosome viscosity probe is as follows:
[0068]
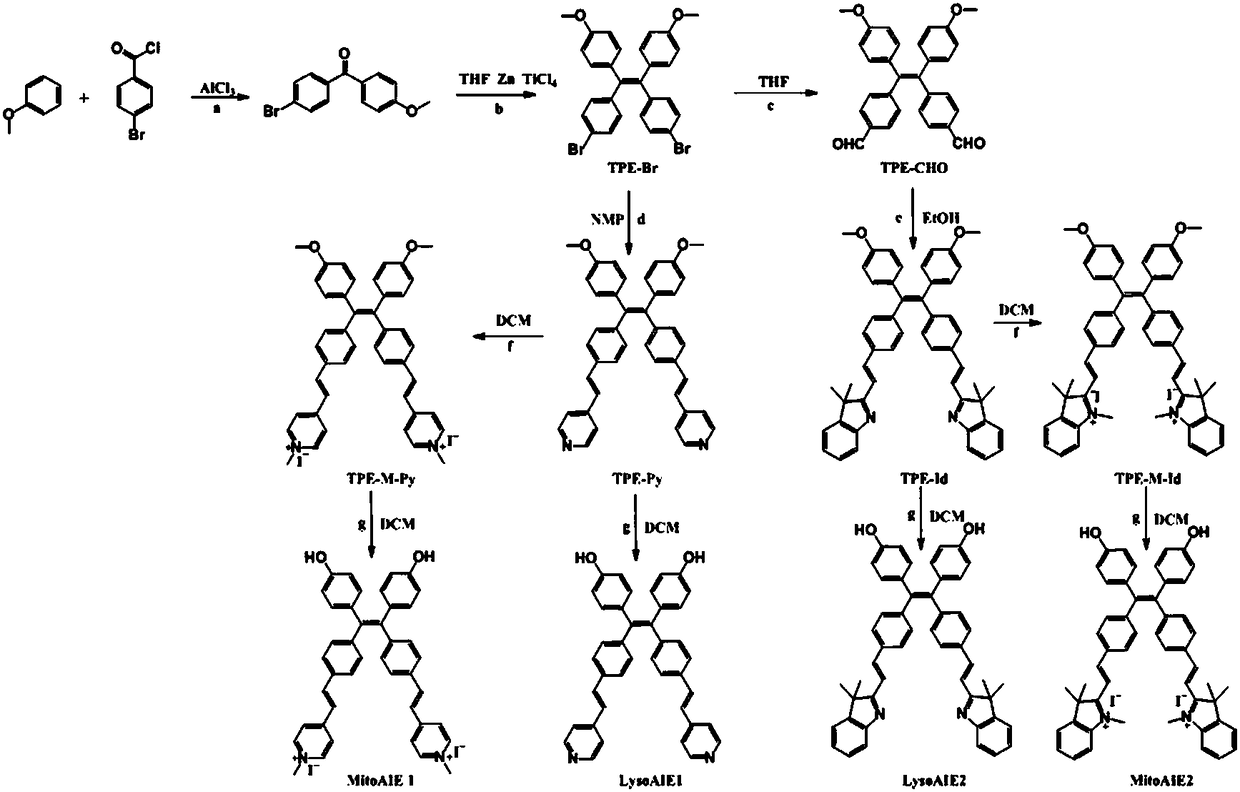
[0069] For the synthetic route of the viscosity probe, see figure 2 .
[0070] 1, the synthesis of (4-bromophenyl) (4-methoxyphenyl) ketone
[0071] Dissolve 2.52g of 4-bromobenzoyl chloride and 6mL of anisole in 20mL of dichloromethane, and stir for 25min under ice-cooling. Then add 1.57g of aluminum chloride to the reaction solution, stir at room temperature for 13h, then pour ice water into the mixture, extract with water three times, filter, and distill under reduced pressure. The crude product is separated by column chromatography to obtain compound (4-bromo Phenyl)(4-methoxyphenyl)methanone,...
Embodiment 2
[0099] The preparation method of the subcellular organelle viscosity probe comprises the following steps:
[0100] 1, the synthesis of (4-bromophenyl) (4-methoxyphenyl) ketone
[0101] Dissolve 2.80g of 4-bromobenzoyl chloride and 8mL of anisole in 20mL of dichloromethane, and stir for 40min under ice-cooling. Then add 2.0g aluminum chloride to the reaction solution, stir at room temperature for 9h, then pour ice water into the mixture, extract with water three times, filter, and distill under reduced pressure, the crude product is separated by column chromatography to obtain compound (4-bromo Phenyl)(4-methoxyphenyl)methanone, yield 50.2%. 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 7.796 (d, J = 8.8Hz, 2H), 7.624 (s, 4H); 6.968 (d, J = 8.4, 2H); 3.891 (s, 3H) 13 C-NMR (100MHz, CDCl 3 ): 194.45, 163.44, 137.01, 132.49, 131.52, 131.31, 129.73, 126.86, 113.71, 55.56.
[0102] 2. Synthesis of compound TPE-Br
[0103] Dissolve 4.22g of (4-bromophenyl)(4-methoxyphenyl)methanone in 10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com